Introduction
Nau mai hoki mai, hello, and welcome back to ward management.
In our last session, we introduced this topic and discussed the roles of pharmacy staff in the hospital ward environment. This week, we continue to discuss pharmaceutical ward stock and its management.
Prescribing and dispensing for hospital patients
When patients are admitted to a hospital, their medical team reviews their medical history, current condition and the medication the patient takes. Based on this assessment, they create a treatment plan, which may include prescribing medications. The medications are then documented on the patient's medical chart, either electronically or on paper, detailing the drug name, route of administration, dosage and frequency.
The nurses on the ward use the medication chart to select the correct medication from the stock of medicines kept on the ward (ward stock), or they may need to order it from the inpatient pharmacy. They then administer it to the patient according to the documented instructions.
Ward stock
Ward stock refers to the medications kept on a hospital ward or unit to meet the immediate needs of patients under the care of that specific ward. It typically consists of various medications, ranging from commonly prescribed drugs for conditions frequently encountered on the ward to emergency medications and those needed for routine procedures.
For example, a cardiology ward will maintain a stock of beta-blockers, ACE inhibitors and statins, while a respiratory ward will have a variety of bronchodilators and steroids.
Purpose of ward stock
The purpose of ward stock is to ensure that ward staff have easy access to essential medications and supplies needed for patient treatment. It helps streamline the medication administration process and ensures patients receive timely and appropriate care. Without a stock of medication on the ward, each item prescribed for each patient must be individually dispensed, supplied by the pharmacy, and then sent to the ward. This could result in delays in treatment administration and potential disruptions in patient care.
Medication not on ward stock
Medication that is charted for a patient but that is not on that ward’s ward stock is dispensed individually for that patient through the pharmacy computer dispensing program.
In most hospitals, the pharmacist will complete a clinical check of the patient and their medications before dispensing them. This clinical check may involve accessing information from sites such as Eclair about their blood, kidney, and liver function tests.
The responsibility for obtaining the necessary medication from the pharmacy lies with the registered nurse of the patient needing the medication. The nurse will fax/email the patient's chart to the pharmacy and highlight the medication that needs to be individually dispensed. Alternatively, they may request from the electronic prescribing system.
The after-hours/emergency drug cupboard
If a patient is prescribed a medicine that is not available in the ward stock and the hospital pharmacy is closed, then medicines can be accessed via the after-hours or emergency drug cupboard. This cupboard can only be accessed by charge nurse managers and clinical coordinators for urgent after-hours items. Depending on the hospital, they may have a time-coded swipe card to access the cupboard, which doesn’t work during the hours the pharmacy is open.
Some of the medicines commonly stored in the after-hours cupboard are:
- antibiotics
- antiretrovirals
- hyoscine hydrobromide
- adrenaline
- antiemetics.
On-call pharmacist
If the medication is not in the after-hours/emergency drug cupboard, the on-call pharmacist can be rung. The on-call pharmacist has lists of medications on all the wards and can direct the person requiring the medication to the appropriate ward. If all else fails, the pharmacist can come in to open the pharmacy and obtain the medication.
Using the after-hours drug cupboard
It is important to have the after-hours cupboard adequately stocked at all times. This will reduce the number of callouts to the on-call pharmacist and make urgent items immediately available if necessary. Often, the medication will be packed into smaller quantities to reduce wastage.
All items taken from the emergency drug cupboard must be documented with the:
- date the medication was taken
- ward it was supplied to
- drug form
- quantity.
The ward that has used the medication will then be charged for it.
The pharmacy assistant or technician checks the emergency drug cupboard daily and replenishes it as needed.
Effective stock management
It is essential that ward stock is managed effectively and efficiently for the following reasons:
- Medication is a crucial part of a patient’s care: Without access to the necessary medications, patients may experience delays in treatment, worsening of their condition, or even adverse health outcomes. Therefore, supply needs to be reliable and accurate so that a patient’s safety is not compromised.
- Medication is extremely expensive: Inefficient management of ward stock can lead to wastage, overstocking, expiration of medications, or loss of inventory, all of which contribute to increased medication costs. Stock needs to be managed so hospitals can minimise unnecessary costs and allocate their budget more effectively to meet healthcare needs.
Storing stock
To ensure effective and efficient stock management, specific conditions must be met.
- Appropriate storage of medications, which includes enough space for the required stock, the correct environment for both shelf and refrigerated items, and protection from heat and direct sunlight. Incorrect storage not only causes stock loss and higher medicine costs it can also compromise patient safety if medications degrade or become ineffective due to exposure to inappropriate environmental conditions.
- Security of stock to prevent unauthorised access to the medicines, such as lockable access to the ward stock space and a controlled drug cabinet, is essential. Stock loss through theft results in increased medicine costs and compromises patient safety, as it can lead to shortages of crucial medications.
- A stock control system that monitors and analyses changes in stock levels and expiry dates.
- Clear and easy-to-carry-out processes and practices for ordering and processing medications.
- A system to track costs and accurately allocate them to the ward and individual patients.
- A system to reduce the likelihood of health professionals making mistakes when selecting and administering medicines to patients.
Advantages and disadvantages
What do you think are the advantages and disadvantages of maintaining a stock of medications on a hospital ward? Take a few minutes now to jot down your own thoughts before selecting the (+) symbol to compare with our lists.
Maintaining stock medications on a hospital ward is advantageous because it:
- enables healthcare providers to initiate treatment promptly without waiting for pharmacy restocking
- enhances patient safety by minimising delays in medication administration and ensuring timely treatment
- facilitates rapid response to emergencies, ensuring prompt treatment
- results in less faxing/emailing of charts to the pharmacy to order patient medication
- reduces the need for frequent pharmacy deliveries, streamlining medication administration processes
Maintaining stock medications on a hospital ward can be a disadvantage because:
- it relies on nursing staff to accurately select the medication or the correct medication strength from the ward stock for administration
- ward stock can go missing from the ward, thus increasing the ward’s spending when reordering replacement stock
- after the stock has been delivered, it relies on the ward staff to ensure it is stored correctly between pharmacy ward visits
- it requires careful management to prevent stockouts, overstocking, and the expiration of medications
- it occupies valuable storage space on the ward, potentially limiting space for other essential equipment or supplies
- it involves additional costs associated with maintaining and replenishing the ward stock of medications.
Ward stock management systems
The pharmacy computer software system is essential to effective and efficient ward stock management. As you have learned, Toniq or RxONE is the software program used in retail pharmacies. However, the most common software program in the hospital pharmacy is E-Pharmacy, though other systems may be used.
There are two different types of ward stock management systems:
- Ward imprest system: This is carried out using the pharmacy computer software, which includes a ward stock management program.
- Pyxis system: This automated system is used to store and dispense medication. It is integrated with the pharmacy computer software.
This week, we will turn our attention to the ward imprest system and explore how it works as well as its advantages and disadvantages. We will cover the Pryxis system next week. Hoake tatou (let’s go!).
Ward imprest system

The "imprest system" is the method through which a ward's stock of medicines is supplied from the hospital pharmacy to the various wards or units within the hospital. It involves maintaining a predetermined quantity (imprest quantity) of medications based on the specific needs and usage patterns of each ward.
The purpose of the system
The imprest quantity serves as a baseline ward stock level, ensuring that essential medications and supplies are readily available for patient care at all times. It helps prevent stockouts and ensures continuity of care, particularly during periods of high demand or unexpected events.
Maintaining stock
Maintaining the imprest quantity involves regular monitoring of inventory levels, expiry dates, timely replenishment of stock as needed, and adjustments based on changes in patient numbers or treatment needs.
The ward stock is replenished by pharmacy technicians or, more commonly, pharmacy assistants a set number of times a week. Generally, this is between one to three times a week, depending on the type of ward and their requirements. Theatres and the ICU may require more frequent restocking.
Imprest process
Pharmacy assistants and technicians play a central role in the imprest process. These are the steps that you can expect to follow when working in a hospital:
| Step 1 |
Imprest lists are generated through the ward stock management system on the pharmacy computer for each ward.
|
|---|---|
| Step 2 |
The imprest list provides the assistant/technician with the minimum re-order quantity for each item on the stock list.
|
| Step 3 |
The assistant/technician goes from the hospital pharmacy to the wards and physically counts the items in the drug rooms.
|
| Step 4 | The assistant/technician then picks the stock, which another pharmacy technician will check before it is sent up to the wards by a designated person. Depending on the hospital's protocols, this may be an orderly, assistant or technician. |
Extra points
There are some extra points to be aware of during this process, based on when the medication is required, what the medication is, and who is involved.
When
- If an imprest item is urgently required (i.e. it is not a day when the pharmacy staff have come to check the stock), a nurse will send an order down to the pharmacy. This is again picked and checked by pharmacy staff. Depending on the urgency, the nursing or pharmacy staff may take the medication to the ward.
What
- Some items on the imprest list need to be checked with extra vigilance, such as the cardiac resuscitation boxes. If any stock is missing from them, they need to be replaced as soon as possible. Often, a whole new box will be sent up, and the old one will be returned once the new box has arrived on the ward.
- During the stock checking process, the assistant/technician should look for any stock in the cupboard that is not on the imprest list and bring these items to the attention of the ward pharmacist. They may be the patient’s own stock that the ward staff are using, so removal is not recommended without the approval of the ward pharmacist or the nurse in charge.
- Any medication labelled for individual patients should not be removed from the ward until the ward pharmacist or the nurse in charge has confirmed the patient is no longer on the ward or no longer requires it. Usually, there is a section for patient's own medications who are still on the ward and a return section.
Who
- Imprest lists are reviewed regularly between the charge nurse manager and the ward pharmacist using a report generated from the pharmacy software of imprest and dispensed times. Stock levels can also be reviewed earlier, for example, if prescribing practices and drug needs change (which happens regularly with funded brands on the Hospital Medicines List).
- Pharmacy assistants and ward technicians may be instrumental in alerting the pharmacist of the need to change imprest levels.
Ward stock storage and expiry
It is important that ward stock of medicines are stored appropriately and that expiry date monitoring and management protocols are implemented to ensure the safety and efficacy of medications.
The nurses generally maintain the drug storage areas. If stock has been stored incorrectly, such as a fridge medication not being in the fridge, the assistant/technician should either put it in its correct location or, if necessary, replace it. If unsure about the product’s stability, the pharmacist should be consulted.
Managing medication’s expiry dates
Nurses and pharmacy staff are required to check expiry dates of medication regularly.
Here are some examples of how expiry dates are managed:
- The shortest-dated stock is rotated to the front of the shelves so it is used first before the longer-dated stock.
- Short-dated stock (less than three months) is stickered, and alerts on short-dated stock are also put on the imprest list.
- Medication that is due to expire within three months is sent to those wards that have a high turnover of that medication. In this instance, the first ward is credited back the cost of the medication.
- Once a month, pharmacy staff remove and replace stock that expires at the end of the month.
Using the ward imprest system
Let's consider the key points to remember when using the ward imprest system. As some of the sentences in the following activity are incomplete, find the answers in the content above or use educated guesses to fill in the blanks. This activity is designed to get you thinking about each point, so it's okay if you don't get all the answers correct. Use the 'Show solution' option to see the answers you missed, and be sure you understand these key points of the imprest system before moving on.
Advantages and disadvantages
The ward imprest stock management system has advantages and disadvantages. Āta whakaaro (think carefully) and jot down at least two advantages and disadvantages before you expand the labels to compare your answers.
Advantages of the ward imprest system include the following:
- Timely access: Ensures that essential medications and supplies are readily available for patient care on individual wards, reducing delays in treatment.
- Decentralised management: Allows for ward-level control and management of stock, enabling nurses and healthcare professionals to respond quickly to patient needs.
- Flexibility: Provides flexibility in adjusting stock levels based on changes in patient numbers, treatment protocols, or supply availability.
- Streamlined workflow: Streamlines the medication administration process by reducing reliance on central supply departments and minimising medication access delays.
- Patient safety: Enhances patient safety by ensuring that medications are readily available and administered promptly, minimising the risk of treatment delays or interruptions.
Disadvantages of the ward imprest system include the following:
- Inventory management: Requires diligent monitoring and management to prevent stockouts, overstocking, expiration of medications, or loss of inventory.
- Storage space: Occupies valuable storage space on the ward, potentially limiting space for other essential equipment or supplies.
- Accountability: Increases the responsibility of ward staff for managing and maintaining accurate records of inventory transactions, which can be time-consuming.
- Cost: Involves additional costs associated with managing and replenishing ward stock, including procurement, storage, and inventory management.
- Risk of errors: It relies on the nursing staff to accurately select the correct medication from the ward stock for administering to the individual patient.
Ward imprest system summary
Tino pai tō mahi, good work. This was a lot to take on board! To consolidate what we have been learning about the ward imprest stock management system, complete the following six-question pātaitai (quiz).
Self-directed learning activity
Choose your own (SDL) activity! This week, your focus is on ensuring that you are up to date in your learning and that you feel confident with the content we’ve covered in this module, specifically:
- Communication
- Communicating appropriately and effectively with other healthcare professionals.
- Collaborating effectively with other healthcare professionals.
- Ward management
- The role of pharmacy staff in hospital wards.
- The management of medication for patients in hospital wards.
Activity ideas
Here are some ideas to get you started. You may wish to complete all these activities, or you may want to focus on one or two. Remember, you have the steering wheel on your learning journey, so it’s up to you where you want to go!
- Reflect on the stock management practices in community pharmacy, noting the practices and principles that apply to ward stock management. Share at least one of these practices or principles in the forum: SDL: Stock Management.
- Review relevant textbooks, articles, and websites to extend your learning. Share the following in the forum:SDL: Share A Resource.
- One resource (relevant to the topics we have covered) that you found interesting or helped you to understand a concept.
- Explain why you chose to share this piece of information. For example, “This YouTube video helped me visualise the impact that small non-verbal cues, like folding my arms while I speak, can have when communicating with a colleague. As it can make me look disinterested or impatient, I’m now aware that this is something I can work on.”
- Seek answers to any questions you may have. Remember, your pharmacy colleagues and tutor are awesome sources of support and information.
- Review your previous online learning sessions and update your personal notes and glossary.
Mahi pai, good work! You have completed another week of Professional Practice 2. Next week, we'll continue exploring ward stock management, shifting the focus to the Pyxis ward stock management system.

Afio mai, welcome to Dispensing 2 and our topic on aseptic compounding. Tenei wiki, (this week), we learn more about contamination control and the role of clean rooms. We will also identify different quality control measures and how to use them to ensure compounded medicines are safe for patient use.
Journal post
Aseptic Review
Let’s start by bringing your previous learning to the surface. Consolidating and reviewing this information creates a firm foundation for this week’s learning.
- Create a new journal post titled ‘Aseptic Review’.
- Answer the following questions.
- Publish to ‘All course users’.
- Save the permalink to your Index of Journal Posts.
Questions
You are preparing for aseptic compounding…
- …and you have just completed aseptically gowning. What do you do next and why?
- …and you have just completed aseptically gloving. What do you do next and why?
Note: If you’re unsure how to answer these questions, chat with a pharmacy colleagues or online tutor for guidance.
Clean rooms
Last week, you became comfortable with a crucial element of contamination control, which involved donning PPE correctly. Another crucial part of the aseptic compounding process is using “clean rooms”. A "clean room" goes further than being just a tidy room! It refers to a highly controlled environment specifically designed to minimise the risk of contamination during the preparation of sterile medications.
The rooms are carefully engineered to meet strict standards, ensuring that airborne particles, microorganisms, and other contaminants are minimal. To visualise this, scroll back up to the image at the start of this topic.
Clean room design
One of the most important aspects of maintaining a sterile, clean room is ensuring that the filtered air flows consistently across the surfaces within that space. The design of the clean room directly impacts this.
There are two clean room designs:
- unidirectional (vertical or horizontal)
- non-unidirectional.
In basic terms, this means that the airflow either moves in one direction (unidirectional) or multiple directions (non-unidirectional).
To view examples and find out more about each of these clean room types, expand the following labels.
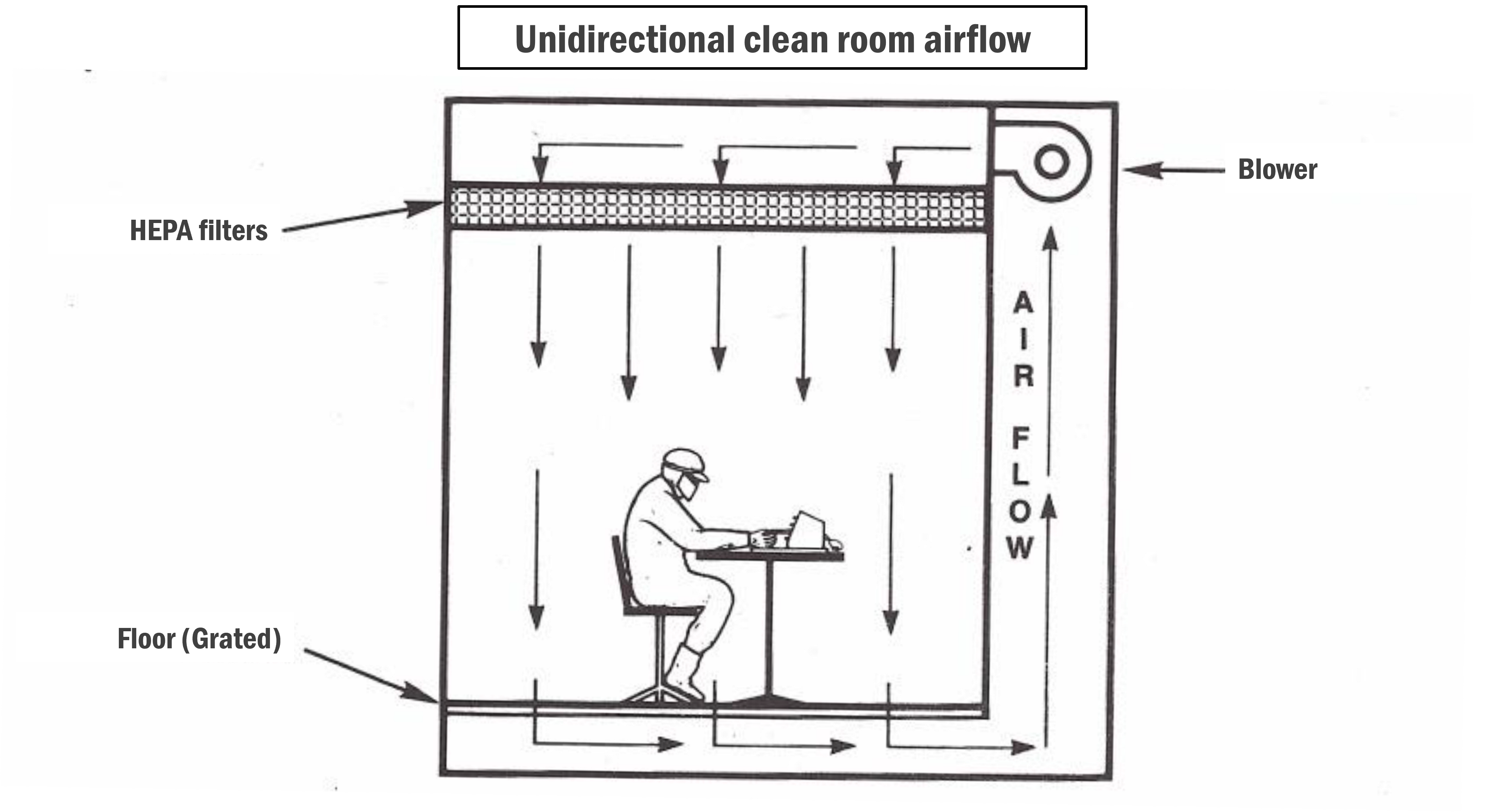
- In a unidirectional clean room, airflow moves in a single direction, either vertically (from ceiling to floor) or horizontally (from one side to the other). This controlled airflow helps minimise the risk of cross-contamination by directing airborne particles away from the critical work area.
- Devices such as HEPA (High-Efficiency Particulate Air) filters and HVAC (Heating, Ventilation, and Air Conditioning) systems control the airflow. HEPA filters remove airborne particles, while HVAC systems regulate temperature, humidity, and airflow direction.
- As the names suggest, vertical unidirectional clean rooms typically have ceiling-mounted HEPA filters that supply clean air downward (vertically), while horizontal unidirectional clean rooms have side-mounted filters that supply clean air across the workspace (horizontally).
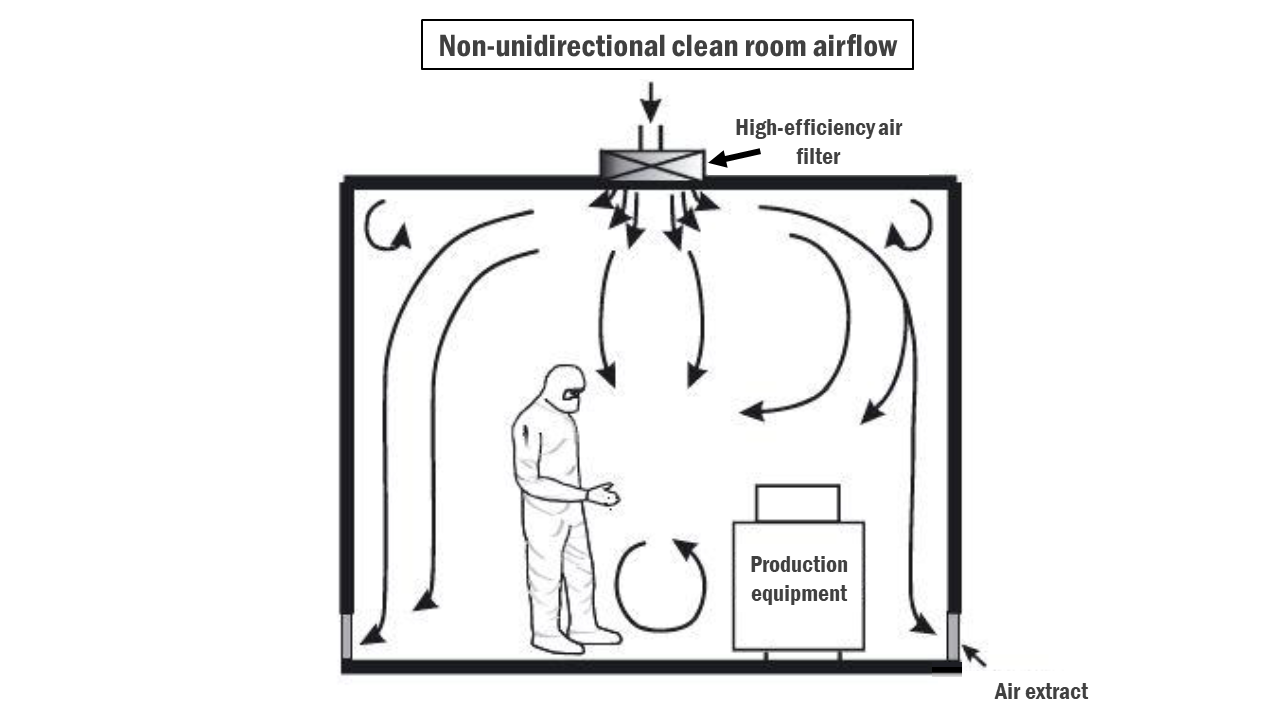
- In non-unidirectional clean rooms, airflow may move in multiple directions rather than a single direction. This may include turbulent airflow patterns.
- Similar to unidirectional clean rooms, non-unidirectional clean rooms utilise HEPA filters and HVAC systems to control airflow. However, the airflow may be less strictly controlled compared to unidirectional clean rooms.
- Filters may be distributed at equal intervals throughout the room or grouped in critical areas.
Hospitals may use a non-unidirectional clean room design for aseptic compounding when the requirements for compounding sterile preparations are less stringent, and the risk of contamination is deemed acceptable based on risk assessment.
Here are some scenarios where non-unidirectional airflow clean room design might be used:
- Low-risk compounding: When compounding low-risk sterile preparations, such as simple IV admixtures or oral liquid medications, some pharmacies may opt for non-unidirectional airflow clean rooms. These preparations have a lower risk of microbial contamination compared to high-risk preparations.
- Limited resources: Due to space or budget constraints, some hospital pharmacies may be unable to install or maintain unidirectional airflow systems. In such cases, non-unidirectional airflow clean rooms may be used as an alternative.
However, regardless of the airflow design, it's essential to note that even in scenarios where non-unidirectional airflow clean rooms are used, strict adherence to cleanliness protocols and regulatory guidelines is necessary to ensure patient safety and prevent contamination of sterile medications.
Designing for clean rooms
When it comes to clean room design, the following best practice guidelines should be adhered to:
- Airlocks must be implemented to separate clean areas from adjacent dirty areas.
- Horizontal surfaces should be regularly cleaned as they are prone to microbial buildup.
- The construction materials used should be made for easy cleaning and disinfection.
- Equipment placement should not obstruct airflow or interfere with airflow patterns.
Clean room grading
In New Zealand, clean rooms are graded on their standard of cleanliness using a classification system based on letter grades A, B, C, and D. The grade is based on the number of particles present per cubic metre of air allowed at rest and when products are being made. Grade A is the most sterile, and grade D is the least sterile.
Clean room parameters
As you can imagine, it is crucial that the environment inside a clean room is well-controlled, reliable, and predictable. This ensures that conditions are optimal for aseptic compounding and ultimately maintains the sterility of pharmaceutical products.
To achieve this, these different environmental parameters must be controlled:
- Air quality
- Airflow
- Temperature
- Humidity
- Pressure differentials
- Surface cleanliness
- Gowning and personnel practices
- Equipment sterility
Put your brain to the test and match each environmental parameter with its description in this two-question pātaitai (quiz). You will be familiar with some of these already, but make sure to add any descriptions that are new to you to your notes or glossary.
Laminar airflow

Did you know that sneezing produces between 100,000 – 200,000 aerosol droplets, which can attach to dust particles and be present in the air for days or even weeks? Yikes. As environmental air can be highly contaminated (achoo!), laminar airflow is an important method used to control this.
Inside the clean room, aseptic compounding is usually carried out in these special work zones called laminar airflow hoods or isolator cabinets, which you can see in the image above. These zones help to control environmental air, almost like a (very) fancy extractor fan that most people have in their kitchens to remove the smoke and steam from the culinary delights cooking on the stovetop.
How it works
This is how a laminar airflow hood works:
- Room air is taken into the unit and passed through a pre-filter to remove contaminants such as lint and dust.
- The air is then compressed and channelled up behind and through the HEPA filter (High-Efficiency Particulate Air filter), which removes all particles, even as small as 0.3 microns (the smallest of bacteria!).
- This clean, filtered air flows in one direction over the critical workspace. It is produced at a constant and positive flow and at a velocity (speed) that prevents room air from entering.
Types of laminar flow
There are two different types of laminar flow:
- Horizontal
- Vertical
Although these names provide a hint as to how each type works, read on to find out more.
Horizontal:
In this type of hood/cabinet, the filters are at the back of the unit, and the air streams horizontally over the critical zone towards the operator.
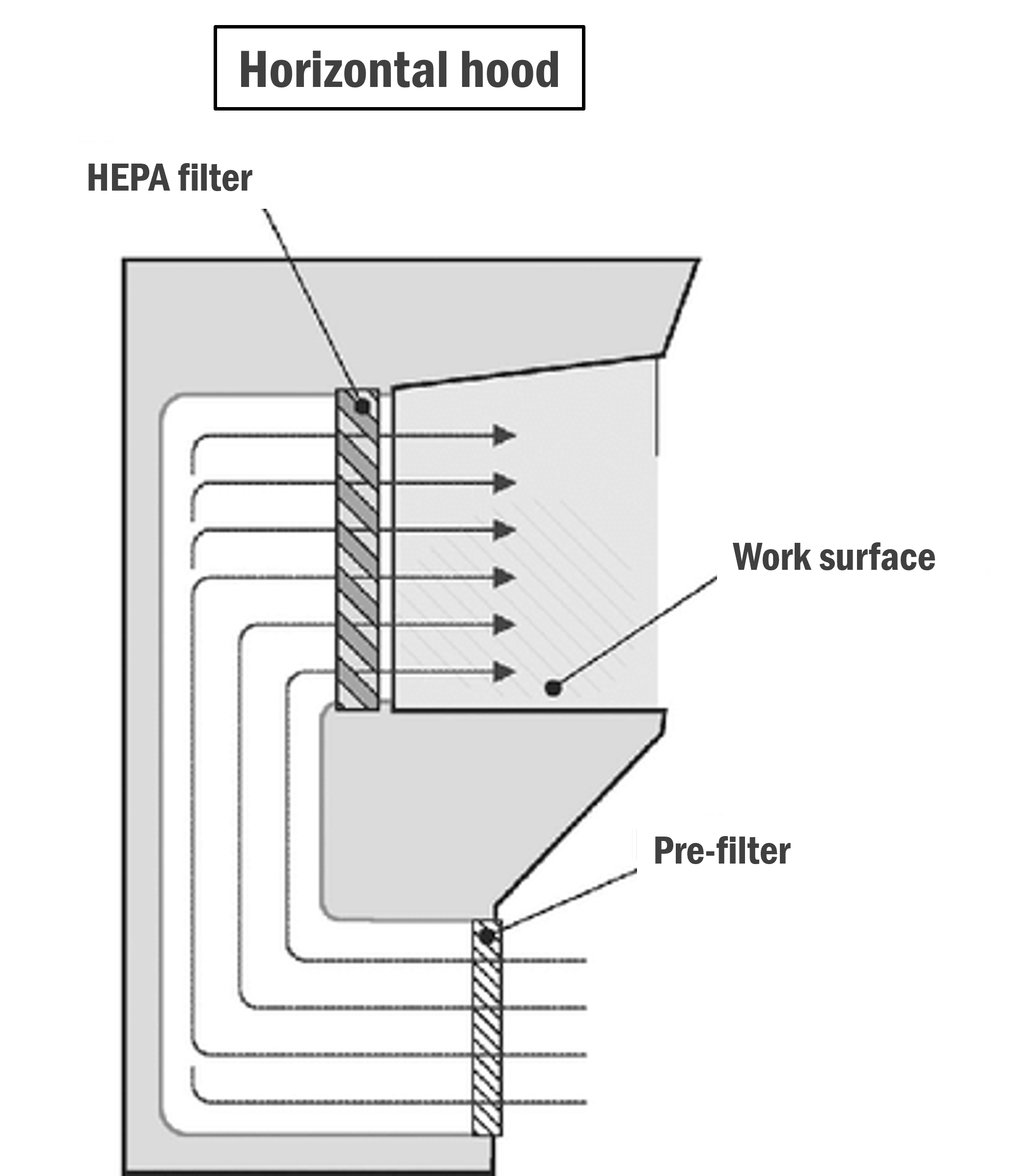
Image: Research Gate
Vertical :
In this type of hood/cabinet, the filters are above the work surface and force clean air downwards in vertical parallel streams.
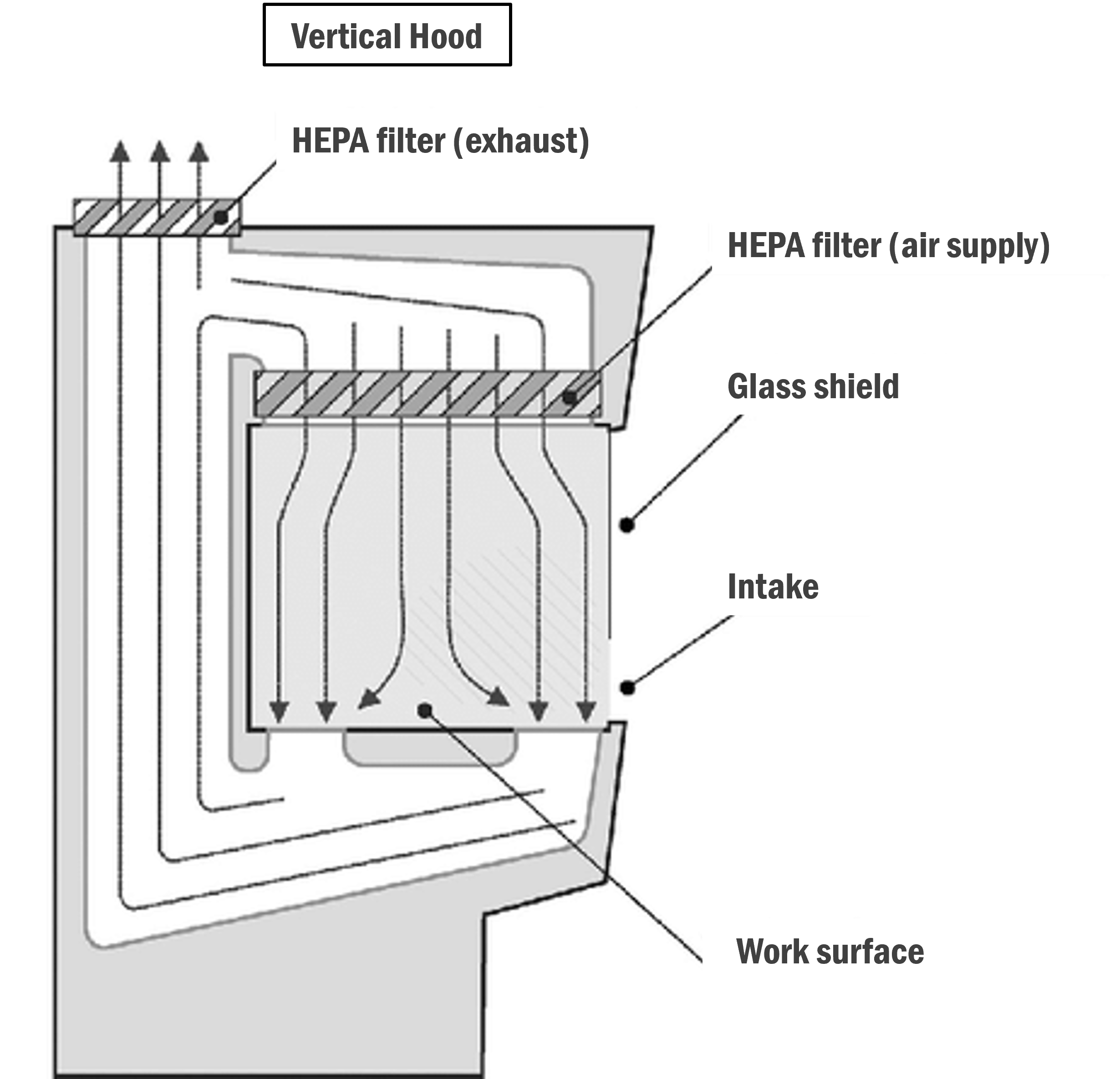
Image: Research Gate
Benefits
The main benefits of using laminar airflow hoods include that:
- they provide clean air to the working area
- they provide a constant airflow out of the work area to prevent room air from entering
- the air flowing out from the hood suspends and removes contaminants introduced into the work area by personnel.
Working in a laminar airflow hood
These are some basic rules to follow when working in a laminar airflow hood:
- Airflow from the HEPA filter must never be obstructed.
- Hands should never obstruct airflow around the area where the needle enters the vial or ampoule.
- Items should be placed as close to the air source as possible.
- Do not spray or squirt solutions onto the HEPA filter.
- Minimise movements as much as possible inside the laminar airflow.
Compounding isolators

Image: LABRepCO
Compounding isolators are similar to laminar airflow cabinets in that they are a specialised work zone inside a clean room. However, isolators are enclosed zones that offer increased protection for both operators and products. They have a glove-sleeve system that allows compounding personnel to access the interior workspace while maintaining sterility. This system typically includes gloves integrated into the enclosure walls and sealed access ports for compounding personnel to insert their hands.
Explore
In the image above, you can see a compounding isolator. If you’d like to see this in action, watch this short YouTube clip: Sterile Compounding of a Gentamicin Bag.Types of isolators
The two most commonly used isolators are:
- Positive pressure isolators: These isolators protect both the product and the operator.
- Negative pressure isolators: These isolators give greater operator protection but are less protective for the product. They are essential when cytotoxic products are prepared and are used to increase protection for the operator.
Quality control systems
The quality control systems for aseptic compounding refer to the comprehensive procedures and protocols implemented to ensure the integrity, safety, and efficacy of compounded medications. Without these in place, there’s a larger risk of microbial contamination, which could lead to serious consequences such as patient infections, compromised health outcomes and legal liabilities.
“Quality control systems” is a broad term that covers a lot of different aspects. However, we will cover the following:
- Operator technique
- Operator validation
- Documentation
- Quality checks
- Error monitoring
Operator technique
The operator's correct technique to achieve product sterility is known as the aseptic technique. It plays a pivotal role as a quality control measure by reducing the risk of microbial contamination, which might otherwise compromise the integrity, efficacy, and safety of compounded products.
The most basic principle for the aseptic technique is known as the “no-touch” technique, which emphasises minimising direct contact with sterile components to prevent contamination.
Key points
When using the aseptic technique inside the clean room and laminar airflow cabinets, you must do the following:
- Maintain sterility: Ensure that all surfaces, equipment, and materials within the clean room and laminar airflow cabinets are sterile before beginning the compounding process.
- Position work strategically: Place work mid-air whenever possible within laminar airflow cabinets to maintain unobstructed airflow and sterility.
- Avoid contamination sources: Keep fingers, loose clothing, and other potential sources of contamination away from critical zones within the working area.
- Follow proper procedures: Follow established protocols for compounding within clean rooms and laminar airflow cabinets, including using sterile gloves and other personal protective equipment (PPE).
- Handle equipment correctly: Position needles, syringes and vials mid-air and point them upwards within the airstream.
- Uncapped needles should not have contact with any surface.
- When removing air from a vial, avoid aerosols or droplets being formed.
- Hang large containers or IV bags from the rail inside the cabinet to gain the advantage of the airflow.
Operator validation
Operator validation ensures that compounding personnel have the required skills, knowledge, and training to uphold the sterility and safety of compounded medications.
Verifying competency
To ensure that they are competent in performing aseptic techniques and procedures, operators may undergo specific practical tests or assessments to assess their proficiency in aseptic compounding techniques.
These may include the following (interestingly named) tests:
- Finger dab: This test assesses the operator's handwashing technique by examining the fingertips of gloves worn during the compounding process. The operator must touch their gloved fingertip onto a sterile agar plate. The agar plate is then cultured to detect any microbial contamination.
- Broth transfer: This test assesses the operator's aseptic technique. The operator carries out the aseptic transfer of a sterile broth from one container to another using a sterile implement. The transferred medium is then cultured to detect any microbial contamination.
Documentation
Documentation plays a crucial role as a quality control system in aseptic compounding by providing a comprehensive record of the compounding process. It creates a complete set of records and establishes an audit trail for all activities, including the work performed, materials utilised, and personnel involved in the process.
By carefully documenting each step of the compounding procedure, errors can be minimised, and corrective actions can be promptly implemented if necessary. Documentation also serves as a valuable resource for investigating incidents or resolving issues.
As you can see, most of the following documentation types used will be familiar to you at this stage of your studies:
- Standard Operating Procedures (SOPs)
- Batch manufacturing records
- Product specification documents
- Staff rules and policies
- Equipment performance checks
Quality checks
Quality checks are another quality control system. Checks are conducted at various stages of the compounding process and are meticulously documented. These checks begin with examining materials before assembly and continue at each subsequent step until the final release is authorised. By implementing checks throughout the entire compounding process, any issues or deviations can be promptly identified and addressed, minimising the likelihood of errors or contamination.
Error monitoring
Error monitoring must be carried out to ensure the quality of aseptic compounding. Error monitoring involves identifying and fixing issues or discrepancies in the compounding process. By detecting and addressing errors promptly, this process can prevent potential risks to patient safety and product quality.
Error monitoring typically includes methods such as visual inspections, automated monitoring systems, documentation review, and staff training to ensure adherence to protocols and minimise the occurrence of errors.
Common errors include:
- mistakes in transcription
- calculation errors
- inaccuracies in drug selection, dosage, or strength
- improper dilution of infusion fluids
- incorrect final volume
- labelling errors or using the wrong container
- inaccurate expiration dates.
Given the number of potential error sources in aseptic compounding, it is vital to record all errors and near misses. In the healthcare industry, quality improvement is especially important. If healthcare workers repeat the same mistakes, the safety of patients is at risk. That’s why providing staff with a list of common errors is a proactive way to raise awareness and prevent the errors from happening again.
Environmental monitoring
Environmental monitoring is an important aspect of quality control. This involves testing equipment, cleanrooms, and personnel to ensure that:
- clean rooms, laminar flow units, and isolator cabinets function according to specifications
- facilities and equipment maintain the environmental conditions within acceptable limits
- SOPs are being adhered to to preserve the integrity of the cleanroom environment.
Legislations and standards
Quality control is also achieved by strictly adhering to the legislation and standards for aseptic compounding. These include:
- Health and disability services Standards - Pharmacy services Standard: NZS 8134.7:2010
- Medicines Act 1981
- Medicines Regulations 1984
- Current internationally recognised standards for good manufacturing practices of aseptic compounding.
Audits are carried out to ensure these standards are met. These include internal audits, which are carried out by staff, and external audits, which staff independent of the unit carry out.
Important
Please be aware that legislation and standards governing aseptic compounding may change due to evolving regulatory requirements and advancements in pharmaceutical practices. This means that pharmacists and pharmacy technicians must stay updated and adapt to these changes to maintain compliance and ensure the highest standards of safety and quality.Knowledge check
Ka pai, well done. Now that you've completed reading about clean rooms and quality control systems use your brain in a different way to complete this nine-question quiz to challenge your understanding of these concepts. Karawhiua, give it your best shot!
Self-directed learning activities
Once you finish this course, your learning doesn’t stop. As you continue in your career, it’s crucial that you keep up to date with best practices to ensure a high standard of professional competence and, ultimately, to provide the best patient outcomes. It is so important that these are two of the principles highlighted in the Pharmacy Council’s Code of Ethics!
Keeping this in mind, in this week's optional activities, we challenge you to critically analyse how you can use AI as a tool for self-directed learning and professional development.
Activity 1
Watch: Using of syringes, needles, opening vials […] (13:43 minutes)
Mātakitaki, watch this video to summarise the topic of aseptic compounding.
Despite being five years old and originating from the United States, it provides a valuable summary of the information we have discussed and is from a reputable source. It includes demonstrations and explanations of aseptic handling techniques for syringes, needles, and filters within a laminar airflow cabinet. While specific SOPs may vary between hospitals, the underlying principles remain consistent.
activity 1 - optional extra
To celebrate nearing the end of the course, we want to show how you can leverage independent learning tools and AI to extend your learning, elevate your study and have some fun!
Watch this screencast for our example (using the Aseptic Techniques video above), then have a play yourself before critically analysing how you can use tools like these creatively and safely.
Note: If you find the screen too small, you can select the icon on the bottom right of the video to make it full-size.
Kei a koe te rākau ināianei, it's your turn now! Experiment using your own notes and resources, or click the (+) symbol to have a play using the tools from our example.
- Copy the YouTube video transcript. Transcript
- Paste the transcript into ChatGPT to remove the timestamps: ChatGPT
- Upload the content into Quizlet to generate your learning materials: Quizlet
To help you analyse the AI tools and learning strategies we've shared, answer the following questions in this week's forum: SDL: Activity 1 - Optional Extra.
Questions
- What are some potential pitfalls of using AI tools like Quizlet and ChatGPT?
- How can you ensure the content is reliable, accurate and relevant to New Zealand?
- What do you do if AI tools like Quizlet or Chat GPT give you the wrong information?
- What are some of the advantages of these tools?
- Do you use tools like these? If so, share your experiences using them.
Make sure to check out your peers' posts for different perspectives, thoughts and experiences.
Activity 2
To prepare for next week's topic, revise your previous learning on the parenteral route of administration.
Here are some questions to structure your revision:
- Why may medications be given via this route?
- What are the advantages and disadvantages of this route of administration?
- What medications or products are administered via this route?
Activity 2 - Optional extra
Practice using the tools and/or learning strategies we shared in the previous activity to help you revise.
As we often learn best from each other, feel free to share the learning strategies or tools you used in the forum. You may also like to share your (fact-checked) learning materials (such as summarised notes, quizzes, or flashcards): SDL: Activity 2 - Optional Extra.
Congratulations, you have now completed our topic on aseptic compounding. Our upcoming session will introduce a new topic (and acronym for your glossary), Total Parenteral Nutrition (TPN).
