Haere mai, welcome back to another week of study!
This week, we will continue covering stock management and the reconstitution of oral medicines, and we will also dive deeper into pathogenic viruses.
Welcome back to our topic on stock management.
In this lesson, we focus on the topic of storage. Correct storage of medicines in a pharmacy is essential. All pharmacy staff should be trained in the appropriate storage of the pharmaceutical products held on their premises.
Storage of medicines
Take a moment now to pause and write down a list (on a scrap of paper or digitally) of what you think the potential consequences are of storing medicines improperly.
Once you've done that, click on the (+) button to compare your thoughts with the answers.
Answers:
Exposure to heat, light, or humidity can lead to the breakdown or deterioration of active ingredients, reducing the medication's efficacy and therapeutic effects.
Temperature above or below the recommendations can cause changes to the medicine's strength, resulting in inaccurate dosing and inadequate treatment outcomes.
Some medications are susceptible to spoilage or decay when exposed to certain environmental conditions leading to changes in smell, discolouration and changes in consistency.
Degraded or altered medications may lead to unexpected side effects or adverse reactions in patients that can compromise their well-being.
Inadequate storage can increase the risk of contamination from external factors, such as dust, microorganisms, or other substances, compromising the medication's safety and sterility.
Improper storage can shorten the medication's shelf life, resulting in medications becoming unusable before their labelled expiration date.
In some cases, improper storage may lead to the need for discarding medications, resulting in wastage and increased costs for the pharmacy.
Improper storage practices may lead to non-compliance with legislation and regulatory standards. This could result in legal consequences for the pharmacy.
Patients may lose confidence in the pharmacy's ability to provide safe and effective medications if improper storage practices are evident.
Ka pai tō mahi - good work! Did you come up with a similar list?
Where and how medicines are stored
When it comes to where and how medicines and related products are stored in a pharmacy, the following factors need to be considered. Click to read more about each of these factors.
Is it a:
• controlled drug?
• prescription medicine?
• pharmacist only medicine?
• pharmacy only medicine?
• Over The Counter (OTC) medicine?
• a hazardous substance?
Are there legal requirements under the medicines and misuse of drugs legislation that need to be followed?
Based on the chemical and physical properties of the medicine, what are the storage recommendations given in the data sheets? For example, store at room temperature.
Is this medicine or product a potential hazard? For example, is it flammable?
What are the practices, procedures, and pharmacy standards for medicine storage?
Legal requirements for medicine storage
In this activity, you will discover some of the legal requirements for medicine storage. Use the search function on the New Zealand Legislation website to answer the following five questions:
The physical storage requirements for medications that do not need refrigeration are as follows:
- Store at temperatures below 25℃.
- Keep away from direct heat.
- Keep away from direct light.
- Keep away from moisture.
At this point in your journey towards becoming a pharmacy technician, you have gained a lot of valuable knowledge about Controlled Drugs (CDs). Let’s review some key points regarding the unpacking and storing of CDs:
- When unpacking a stock delivery of CDs, you will check the stock received against the invoice.
- The CDs are then taken into the dispensary, where they are checked off by a staff member and entered into the CD book.
- The invoice is stamped and signed (CD signing sheet) for return to the wholesaler.
- The CD register is countersigned by the pharmacist.
- The items are placed into the safe, which is securely locked.
Storage of hazardous substances

Now, let's turn our focus to hazardous substances and how they are stored. In general, a hazardous substance is something that, when swallowed, inhaled, injected, or otherwise absorbed by the human body, is likely to destroy life or be injurious to health.
A hazardous substance may have one or more of the following properties:
- Explosiveness: The ability of a substance to release energy suddenly and violently, resulting in an explosion.
- Flammability: The ease with which a substance can catch fire or ignite when exposed to heat, sparks, or an open flame.
- A capacity to oxidize: The ability of a substance to react with oxygen, leading to a chemical reaction that can release heat or promote fire.
- Corrosiveness: The property of a substance to cause damage or deterioration to materials it comes into contact with, including metals and other surfaces.
- Toxicity: The potential of a substance to cause harm, illness, or even death to humans or living organisms when exposed to it.
- Ecotoxicity: The harmful effects a substance can have on the environment and wildlife when released into ecosystems.
GHS PictogramsThe pictograms you see here are from The United Nations Globally Harmonised System of Classification and Labelling of Chemicals (GHS). The GHS provides a standardised approach for identifying and communicating the hazards associated with various chemicals, including pharmaceuticals. You may have seen some of these pictograms before. |
In a pharmacy environment, there are many substances that are recognised as hazardous. Therefore, careful handling and appropriate storage is vital. Hazardous substances in the pharmacy fall into the following groups:
- Corrosive
- Flammable/volatile
- Clinical waste
- Disinfectants
In this next activity, learn more about each of the above groups of hazardous substances.
Journal post
Hazardous substances
- Using your internet searching skills, carry out research into how each of the hazardous substances (corrosive, flammable/volatile, clinical waste, disinfectants) cause harm to the human body.
- Record your findings in your journal. You may like to use this template for recording and posting to the journal and to save it for future reference.
Handling and storage recommendations (hazardous substances)
The recommendations for the handling and storage of hazardous substances will be found in a pharmacy’s SOPs and may include the following recommendations and best practice guidelines:
- When receiving and unpacking hazardous substances, check for any damage or moisture, and if there is, take immediate action as per the SOP for accidental spills.
- Follow the handling and storage instructions on the medicine or product. Some types of hazardous goods require extra conditions. For example, flammable and volatile substances need an area that is also well-ventilated and away from ignition sources.
- Store in the designated area of the dispensary away from other medicines or products to avoid cross-contamination and accidental dispensing with other medicines or products.
- If a hazardous substance is repacked, ensure that all warning labels and storage information are transferred from the original container to the new container.
- Hazardous substances that are displayed in the retail area of the pharmacy should be placed on shelves 1.5 meters above the floor. This is to keep them out of reach of children and avoid accidental spills.
- Do not store or display substances that are on, above or near any other medicines or food-related products.
- In the situation of an accidental spill, take action immediately following the SOP for the particular substance. Each substance may need to be dealt with using a spill kit and procedures tailored for the particular substance.
Actions may include:- Putting on gloves or other PPE to protect the skin and airways from contact with the substance and its vapour.
- Opening a window to provide ventilation from hazardous vapour in the air which can cause breathing problems, headaches, nausea, or other effects.
- Removing any sources of ignition from the area that might cause flammable substances to catch fire.
- Isolating the area to prevent others from coming into contact with the substance.
- Following the SOP for how to dispose of the spilt/waste substance. For example, clinical waste such as sharps are disposed of in the yellow sharps bin.
Cytotoxic medicines
Cytotoxic medicines represent another hazard encountered in the pharmacy. As a pharmacy technician, these medicines will be the most common hazardous substance you will be dealing with. Let's explore the meaning and implications of cytotoxic medicines.
"Cytotoxic" is a term formed by combining "cyto," which comes from the Latin word for cell, and "toxic," meaning harmful or poisonous to living organisms. Consequently, "cytotoxic" signifies something that is harmful to cells. Cytotoxic medicines are used in the treatment of cancer, leukaemia, auto immune disorders, and organ transplant rejection.
Cytotoxic medications are considered hazardous for the following reasons:
- They are toxic to healthy cells as well as unhealthy cancerous cells.
- Improper disposal of cytotoxic medications can pose environmental risks, such as contaminating water sources or soil if not disposed of properly.
- They require specific precautions for handling, dispensing, and disposing of.
- Accidental exposure to cytotoxic medications can have adverse effects on individuals who are not supposed to receive them.
Handling and storage of cytotoxic medicines
Here are the processes that should be followed for handling and storing these medicines:
- When cytotoxic medications are delivered to the pharmacy, they will be in a sealed bag labelled as cytotoxic medication.
- Follow the SOPs for unpacking.
- Store in a designated area in the pharmacy. The equipment used for dispensing cytotoxic medicines should always be kept with the cytotoxic medication in the designated area to minimise the risk of the equipment being used in general dispensing.
- Only use the designated area and equipment for preparation and dispensing. This area should be well-ventilated.
- No direct skin contact with the medicine.
- Follow the pharmacy’s SOPs for handling cytotoxic medicines, including the use of any required PPE.
- Clearly label all cytotoxic medicines with the appropriate warning labels.
- All accidental spills require immediate action following the pharmacy SOPs. This may include isolating the area of the spill so others do not come into contact with the substance.
- Cytotoxic medicine disposal, or disposal of waste from spills, must go into the designated waste containers, separate from other pharmacy waste. This container is collected by a waste management company.
Self-directed learning activity
This week, we would like you to reflect on how important the role of the pharmacy technician is when it comes to medication storage.
- Read this article.
- Share your thoughts in the forum - Week 14 - Professional Practice: Self-directed Learning Activity. You may want to use the following questions to prompt your thinking and structure your post:
- Has this article made you think differently about your responsibilities in stock management? Why, or why not?
- Why do you think stock management is an important part of your role?
- How do you think stock management ensures your customers' well-being?
- Once you’ve published your post, read what your peers have written and respectfully respond to at least one post that you find interesting. Reading other people’s perspectives may challenge or enhance your own thinking.
There was a lot to cover in Professional Practice this week, so to help you summarise and absorb your learning from this week, let’s finish with some fun!
Complete the following seven questions:
Kia pai mai hoki - fantastic, you have now completed week 14 of Professional Practice.
Make sure to complete your self-directed learning activities above. These are designed to summarise, revise and help you retain your new learning. Remember, the more you engage with the content, the easier you will find applying it in the real world!

In this lesson, we continue to explore the practices and procedures for the reconstitution of oral medicines. We move from oral solutions prepared by the customer at home to oral suspensions prepared by pharmacy technicians in the dispensary.
Let’s start this topic with a knowledge check so that you can remind yourself of what you know already. Complete the following five questions.
Now let’s look at the general practices and procedures for reconstitution by working through a scenario.
What Would You Do?
John Smith comes into the pharmacy to get a prescription filled. The prescription is for his 5-year-old son Samuel, who has an ear infection. As Samuel is too young to swallow tablets, the doctor has prescribed an oral liquid. John hands you the following prescription to fill.
You note that this medicine is one you will need to reconstitute from a dry powder. You check the medicine in stock and see that the pharmacy is not very busy, so you inform John that the prescription will be ready in 30 minutes. He tells you he will run some errands and will be back in 30 minutes for collection.
You are now ready to prepare the prescription. Take a moment to think about the environment and hygiene considerations for preparing this medicine. You might like to jot down some notes before reading further.
Click the label or (+) symbol to compare your notes.
- Use the designated space in the dispensary.
- The space should be away from the customer areas and from the direct flow of staff so that you won’t be bumped or disturbed.
- The space should be tidy, uncluttered and well-lit.
- The work surface should be flat, smooth, water-resistant and easily cleaned.

Now, let's put your previous learning into practice!
Reconstituting Augmentin
Augmentin 125mg powder for oral suspension is provided as a dry powder in a bottle. You will add the necessary amount of water to this bottle, resulting in a final total volume of 100mls.
The basic steps to do this are:
Calculate number of bottles required ---> Check the label ---> Pharmacist check ---> Measure water / reconstitute ---> Pharmacist check.
Let's start. Calculate how many bottles of Augmentin you will require to reconstitute enough for the 5-day course.
Number of bottles needed: 1
Now you know how many bottles you need, you will select these from the shelf and check the stock bottle label against the prescription for the following:
- Correct medicine
- Correct strength
- The expiry date of the stock bottle
- That the bottle is sealed and fit for use
Once you have done your check, you will let the pharmacist know that you are ready for them to carry out their check and confirm you have selected the right medicine. This check must be completed before you proceed any further in the reconstitution process.
The instructions for reconstituting the Augmentin are written on the stock bottle. The instructions tell you to add 92 ml of water to the bottle of dry powder.
Let’s revise what you may already know about measuring liquids accurately:
|
 |
What Would You Do?
Back to our scenario: You have selected the correct medicine from stock and have the correct-sized measuring glass.
Now you have gathered your equipment and prepared your workspace, the next thing you should do is...
Ensuring proper handwashing and drying is essential in safeguarding patients' medications. It helps prevent the transfer of harmful microorganisms from your hands to the medication, reducing the risk of contamination with dirt and debris. It also minimises the possibility of cross-contamination between different medicines and products you may have handled recently.
Self-directed Learning
Reconstitution practices and procedures
This week, we would like you to learn about the practices and procedures for reconstitution in your pharmacy workplace. This will involve speaking to your colleagues and observing what information the pharmacy has in place for these processes.
- Record your answers to the following questions in the forum - Week 14 - Introduction to Dispensing: Self-directed Learning Activity.
- Discuss reconstituting practices and procedures with your colleagues to find out more about the process.
- Are there any written SOPs? If so, what do they cover?
- Are there any information signs or checklists on the dispensary walls about reconstituting practices and procedures? If so, what for?
- What medicines does your pharmacy reconstitute, if any?
- What equipment do they use; do they use a conical or a cylinder measure?
- Practice reading the markings on the measuring devices - can you see the graduated markings clearly?
- After answering these questions, was there anything that surprised you? Why? Why not?
- Once you’ve posted your answers, read your peers’ posts to see if anyone has different procedures to your workplace. (By sharing collective experiences, we learn from each other.)
Faufaua (well done!). That’s the end of this topic - you’ve completed another week of Introduction to Dispensing.
Pathogenic viruses
In our previous lessons, we explored pathogenic bacteria (disease-causing bacteria). Now, we turn our attention to pathogenic viruses and begin by looking at some virus facts.
The following are useful resources to watch and read to help you learn about viruses. Once you have looked through the resources, the next learning activity will help you check your learning.
Resources:
- What is a virus? How do they work? - This video is about how viruses infect cells and reproduce, as well as some of the practical uses they have.
- Virus strains - A Science Learning Hub article on the structure of a virus, how they hijack host cells, replicate and mutate. There are very useful short video clips to watch on this page, too.
Activity: All about Viruses
To help you process the facts in the resources above, it’s important to put this information into your own words.
Treatment of viruses
As you know, the best treatment for any health issue is to prevent the health issue from occurring in the first place!
In your own notes, list strategies to prevent contracting a viral infection. This is not only knowledge for yourself but also advice that you will be providing to customers. Once you have completed your list, compare it to the list below:
- Hand hygiene: Wash your hands frequently with soap and water or correctly use hand sanitiser with at least 60% alcohol.
- Clean and disinfect frequently touched surfaces: Regular cleaning of surfaces and objects that are shared and touched frequently, such as doorknobs, light switches, mobile phones, and keyboards.
- Respiratory hygiene: Cover your mouth and nose with a tissue or your elbow when coughing or sneezing. Dispose of used tissues immediately and wash your hands afterwards.
- Avoid close contact: Avoid close contact with people who are sick and maintain a safe distance, especially during outbreaks.
- Stay home when sick: If you are feeling unwell, stay home to avoid spreading the virus to others.
- Avoid touching your face: Refrain from touching your eyes, nose, and mouth, as viruses can enter your body through these pathways.
- Maintain a healthy lifestyle: A strong immune system can help defend against viral infections. Eat a balanced diet, exercise regularly, get enough sleep, and manage stress.
- Wear masks: In situations where viral transmission is a concern, wearing masks can help reduce the spread of respiratory droplets that may contain viruses.
- Vaccination: Vaccines help your immune system recognise and fight off viruses, reducing the risk of infection and severe disease.
- Stay informed: Stay up to date with information from reliable health authorities about viral outbreaks, preventive measures, and vaccination campaigns.
Kei te pēhea koe i te mahi? How did you go? Did you get all these strategies? Did you get any that are not listed here?
Viral infections
Now, let’s take a look at some common viral infections:
Colds and Flus
As you know, colds and flu are caused by viruses. You already know a lot about colds and flu and how to tell the difference between them. You have also explored the pharmacy treatments, advice and when to refer a cold or flu to the pharmacist.
Watch: Flu - What is it? (5:31 minutes)
Watch the following video that focuses on the influenza virus. Although it is an older video that was made before the Covid 19 pandemic, it still provides a valuable summary of the structure of this virus, the different strains, and how it is transmitted, diagnosed, and prevented.
Based on the video you have just watched, answer these questions to check your learning:
Extra for experts!
Remember earlier in this session when we discussed viral mutations and reassortment? What is the difference between them? You may want to scroll back to that activity or look at any notes you have downloaded to remind yourself before you reveal the answers below.
A viral mutation is a small change in a virus and is referred to as a drift.
A viral reassortment is a major change in a virus and is referred to as a shift.
Herpes Virus Family
Herpesviridae is the name given to the family of herpes viruses. We are going to look at two viruses in this family. These are:
- Herpes simplex virus (HSV)
- Varicella zoster virus (VZV)
Herpes Simplex Virus
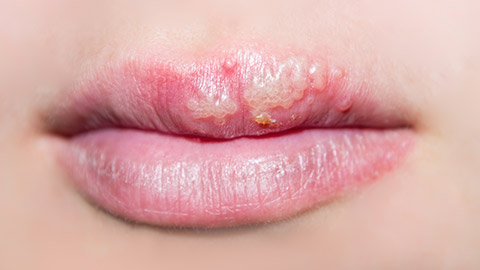
The herpes simplex virus is divided into two types:
HSV type 1:
- Most commonly, it causes infections above the waist, such as cold sores around the mouth and face.
- Treatment involves the use of OTC products.
HSV type 2:
- Most commonly causes infections below the waist, such as herpes of the genitals, buttocks, and anal area. This is a sexually transmitted infection (STI).
- Must be treated by a doctor.
After initial infection with HSV (either HSV1 or HSV2), the virus can establish a lifelong latent (dormant) infection in the body. During this latent phase, the virus travels along the sensory nerves to the nerve roots, where it remains in a dormant or "sleep" state. The virus does not cause any symptoms during this period. Certain triggers, such as stress, tiredness, illness, and exposure to sunlight, can lead to the reactivation of the latent virus. Reactivation results in a recurrence of symptoms.
Varicella Zoster Virus
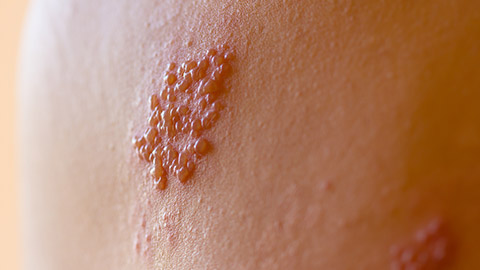
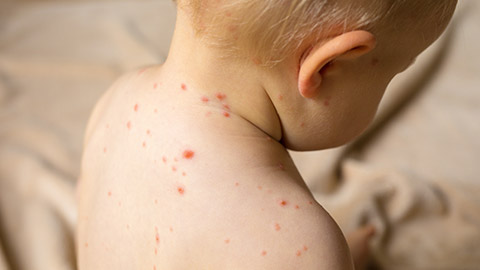
Varicella Zoster Virus (VZV) is the virus responsible for causing chickenpox (varicella). When a person is first exposed to VZV and develops chicken pox, it is referred to as the primary infection. After a person recovers from chickenpox, the virus does not leave the body entirely but instead remains latent (dormant) in the nerve cells near the spinal cord and brain.
During the latency period, VZV remains inactive and does not cause any symptoms. However, in some people, particularly later in life, or when the immune system becomes weakened, the virus can reactivate and travel along the nerve pathways. This reactivation leads to the development of the secondary infection, a condition known as shingles (herpes zoster).
Journal post
Treatment of viral infections
Learning to research and use reliable resources of information is a powerful way to learn. It helps you to understand and retain the knowledge that you need for your role as a pharmacy technician. In this next learning activity, you will research the treatments for cold sores and chickenpox.
Click on this link that will take you to a worksheet with instructions and scenario-based questions to answer.
Vaccination
Previously, we have discussed how the human body defends itself against harmful pathogens. One of the defences we looked at was the specific defence system. The specific immune response provides protection against specific pathogens. This defence system develops after the first exposure to a pathogen and becomes faster and more effective on subsequent exposures to the same pathogen.
Watch: How do vaccines work? - Kelwalin Dharnasarnsombut (4: 35 minutes)
The specific immune response can be triggered when the body is naturally exposed to a virus or when it is deliberately exposed through vaccination. Watch this Ted-Ed video that explains how vaccinations work.
Read the below to learn more about different types of vaccines. Take note of the different patient age ranges for each vaccine type.
| Vaccine Type: | Example: |
|---|---|
Live attenuated vaccine
|
Contains the live but weakened pathogen. Example: Measles, Mumps and Rubella vaccine (MMR). |
Dead (or inactivated) vaccine
|
Contains the dead or inactivated pathogen. Example: Polio vaccine. |
Subunit vaccine
|
Contains only a part of the pathogen that has been made harmless. Example: Hepatitis B vaccine. |
DNA and RNA vaccine
|
Contains part of the pathogen's own genetic code to stimulate an immune response. Example of DNA vaccine: These vaccines are still in development. Example of RNA vaccine: The Pfizer and the Moderna Covid-19 vaccines. |
Self-directed learning activity
MMR Myths
In the late 1990s, a research article published in a medical journal caused a lot of misinformation to be circulated globally about the Measles, Mumps and Rubella (MMR) vaccination. In Aotearoa New Zealand, this triple vaccine is given in a single injection at 12 months and then again at 15 months of age.
Read this blog post from the United Nations International Children’s Fund (UNICEF) that explains the misinformation that surrounding the MMR vaccination, and then complete the questions below. Not only will this activity help you to digest what you’ve learned, but you can save your answers as a handy resource to refer to later.
If you wish to revise your learning from this session on viral infections, we encourage you to check out the following videos:
- Herpes (oral & genital) - causes, symptoms, diagnosis, treatment, pathology
- Chickenpox and Shingles (Varicella-Zoster Virus)
And that’s it for the week! Congratulations on completing Week 14 of Patient Care. It's time to complete your Learning Check-in before moving on to Week 15.