Reading A: The Infection Prevention and Control Team
Reading B: Communication for Patient Safety
Reading C: Hand Dermatitis among Nurses
Reading D: Front Line General Practitioner and The Use of PPE
Reading E: Disinfection and Sterilisation in Healthcare Facilities
Reading F: The Hierarchy of Control and WHS
Important note to students: The Readings contained in this Book of Readings are a collection of extracts from various books, articles and other publications. The Readings have been replicated exactly from their original source, meaning that any errors in the original document will be transferred into this Book of Readings. In addition, if a Reading originates from an American source, it will maintain its American spelling and terminology. AIPC is committed to providing you with high quality study materials and trusts that you will find these Readings beneficial and enjoyable.

Ward, D. (2016). Microbiology and infection prevention and control for nursing students. Learning Matters.
Each NHS organisation has a team of people responsible for IPC. This team has a variety of roles within the organisation, but work together to support its IPC infrastructure and services, reporting to the Trust Board and the chief executive. They are often all members of the IPC committee which meets on a regular basis and produces the organisation's annual programme for IPC activity and annual report to provide information to the Board about where the organisation is in relation to its programme, what healthcare-associated infections have been an issue within the previous year and what actions need to be taken to improve things in relation to IPC. It is a requirement under the Code of Practice which is part of the Health and Social Care Act 2008 (DH, 2015) that healthcare organisations either have or have access to an appropriate mix of expertise in relation to IPC – the IPCT fulfils this requirement. As can be seen here, it is not only NHS organisations which need to have access to such expertise. Non-NHS organisations may have their own IPCT or may contract for the use of a local NHS team, in particular in relation to microbiology advice. The IPCT in the NHS includes people such as the IPC doctor, consultant microbiologist (these two may be the same person) and IPC nurse/ practitioner (IPCN/P).
The Infection prevention and control doctor
Within NHS organisations, someone is designated as the infection control doctor. This might be the consultant microbiologist, public health doctor or infectious diseases consultant. Whoever this person may be, they are seen as the lead for the IPCT. The role of IPCD is not full time but this person often chairs the infection prevention and control committee and liaises closely with the infection prevention and control nurse.
The Infection prevention and control nurse/practitioner
This person may often be the only full-time member of the IPCT. In many organisations there is now a team of nurses within the IPCT; in smaller organisations, such as Mental Health or Care Trusts there may only be one. The IPC nurse/practitioner fulfils a variety of roles including the provision of IPC related advice to all staff within the organisation, clinical audit, risk management, surveillance, staff education, outbreak management and policy and guideline development (Quattrin et al., 2004; Weston, 2013). The IPCP is usually the first point of contact for IPC advice within organisations and is the most visible clinical person within the IPCT. IPCPs work differently within organisations. In some NHS Trusts, IPCPs are allocated to specific divisions so that staff on specific wards have a named member of the IPCT to contact. For example, one IPCP may cover surgery, another medicine, another critical care settings and so on. In other organisations, nurses may take on specific roles so that one IPCN undertakes all the audit, another all the surveillance and so on. Within each of your clinical placements it is worth identifying your point of contact for IPC advice within that area. It has been identified that the presence of an IPCN/P can both improve practice and reduce rates of infection (Venberghe et al., 2002; Ward, 2012).
Jack is a mental health nurse working on an acute ward. One of his patients is admitted after a suicide attempt and this patient has a history of self-harm. There are multiple lacerations on the patient's body, one of which is showing signs of infection. A swab is obtained, and the patient is found to have MRSA. Jack has not had any experience with MRSA for a long time as it is not a common infection on the ward where he works. He is therefore unsure what he should do next. However, he has a good relationship with the infection prevention and control practitioner who covers his ward area and contacts them for advice. The IPCP is able to provide him with information about further screening required to check for colonisation, standard precautions required, whether he needs to be isolated in a side room and treatment options. The IPCP also offers to come to the ward to speak to the patient and his relatives so that they can ask any questions they have about MRSA.
As can be seen from this scenario, one of the important roles of the IPCP is in providing advice and information, to staff, patients and their relatives. They are a vital point of contact for all staff, and it is therefore important to be aware of who the IPCN is for your placement area and how this person can be contacted.
The consultant microbiologist
This member of staff will provide advice related to the medical aspects of patient management. This might include advice about the prescribing of antibiotics, translating laboratory reports, advising doctors on treatment and so on and medically reviewing patients with infections. This consultant may or may not also be the infection control doctor, but will generally be a member of the IPCT and sit on the IPC committee. Outside of the NHS acute hospital Trust setting, the IPC team may function differently. Previously there was the Health Protection Agency which had a nurse and doctor who worked locally, taking responsibility for IPC matters outside the NHS such as in nursing and residential homes. Primary Care Trusts also often employed their own IPC practitioner to cover services such as community nursing, podiatry and dentistry. However, primary and community care provision has changed with the creation of Clinical Commissioning Groups. Community IPC practitioners may now be employed by a variety of organisations such as the CCGs themselves, local councils and acute Trusts who provide IPC services to primary care. This can cause confusion about who to contact for staff working in primary care so good communication within and between services is important to ensure that all staff know who their first point of contact is for IPC. In some areas, health protection or public health nurses may provide IPC input alongside other health protection related responsibilities. Mental Health and Care Trusts function very much like acute Trusts in most cases in relation to IPC provision, employing their own IPC practitioners.
The director of infection prevention and control
This role is required under the Health and Social Care Act 2008 (Department of Health, 2015) and should be in place in all registered NHS care providers. The person undertaking this role is generally not in a full-time or unique role, but it is undertaken alongside another role. However, time should be allocated for the person to fulfil the requirements of this role. The DIPC needs to be an effective leader who is highly visible, senior and authoritative. The DIPC in particular provides assurance to the Trust Board that systems are in place within the organisation to ensure safe and effective healthcare, though they do not need to be a member of the Trust Board. They should, however, report directly to the chief executive.
Considering recent changes within the NHS, the role of the DIPC will differ when working for either commissioning (such as Clinical Commissioning Groups) or provider (such as an NHS hospital) organisations. Provider organisations are expected to have their own DIPC to provide information and assurance to the board. In commissioning organisations, the role will involve providing advice on service specifications and performance indicators related to provider contracts. While there is no single model for how the role of the DIPC is provided within each organisation, the commitment to patient safety and quality care should be paramount. The DIPC role can be undertaken by microbiologists, directors of public health, infection prevention and control practitioners, directors of nursing, medical directors and so on – each organisation will be different.
The infection prevention control link nurse/practitioner
Link nurses/practitioners are used frequently in healthcare settings to support many areas of specialist practice in the UK, including diabetes, pain management and tissue viability. The link nurse role was introduced into infection prevention in 1988 by Rozila Horton; these are practising nurses who have an interest in a specific area of nursing and act as a formal link to the specialist team for that area within the organisation. The role is used in different ways within organisations, with activities and responsibility varying. Titles can be different, requirements to undertake the role can vary and how effective the role is can be dependent on many issues such as the person undertaking the role, how they are perceived by their colleagues and how well they are supported by the IPCT.
Not all link staff are nurses. For example, in departments where nurses are not employed, such as podiatry, the link person might be referred to as a link practitioner. In a basic sense, the role of the link person is to act as a bridge between the IPCT and clinical staff, sharing knowledge and good practice, and sometimes being involved in clinical audits, surveillance and the education of staff in their work area. Four key themes for the role have been identified by the RCN (2012a): acting as a role model for IPC; enabling others to learn and develop their IPC practice; communication and networking around IPC practice; and supporting others in local audit and surveillance, though the last theme is considered optional due to the differing nature of healthcare organisations. Though there is a lack of evidence about the efficacy of link staff, some studies have highlighted benefits to having link systems in place in infection prevention and control (Miyachi et al., 2007; Seto et al., 2013; Lloyd Smith et al., 2014).
In some areas you may be able to clearly identify the person in this role and in others not. You may have also been to practice areas which have no designated IPCLN – this is not a mandatory role and some organisations have taken the decision not to utilise it due to a lack of efficacy in their areas. However, in some areas the use of link nurses can mean that problems are identified and addressed more quickly.
In one town in the North of England there is a link nurse system in the local nursing homes (including adult, mental health and learning disability homes). Some of the homes have an ICPLN/P and some do not. These practitioners have regular meetings with the community IPCP and receive a monthly newsletter. One link nurse informs the IPCP that they have an outbreak of diarrhea and vomiting in Ashwood Court (pseudonym), the adult and dementia care nursing home where she works, which the nurse thinks may be caused by norovirus infection. The IPCP visits the home to meet with the link nurse and offer advice about staff movement, isolation of affected residents, cleaning procedures and staff sickness. The link practitioner works with the IPCP and is in contact with her on a daily basis. The outbreak is over a week later. Two weeks after this, another home which does not have a link practitioner contacts the IPCP to say that they have an outbreak of diarrhea and vomiting which has been ongoing for several weeks, has affected most of the residents and has resulted in seven staff being affected and off sick. As there is no link practitioner, there was no immediate action by the home once an outbreak had been recognised and advice was not sought until the outbreak was widespread affecting both residents and staff.
As can be seen from the above case study, having a link practitioner in place can lead to a quicker resolution to problematic issues such as outbreaks of infection. Having staff with a greater level of knowledge in these areas who have a link and direct relationship with the local IPCP can be of benefit to both patients and staff in such situations.
The infection prevention and control committee
As previously mentioned, NHS organisations generally have an infection prevention and control committee (IPCC) which meets on a regular basis and reports to the Trust Board. Its members vary between organisations but will include the members of the IPC team and other people such as the Chief Nurse, Medical Director, Occupational Health Lead, Health and Safety and Clinical Governance representatives and other people in senior roles within the organisation. The role of the IPCC is multi-faceted and will include planning, monitoring, evaluating, updating and educating in relation to IPC. It sets general IPC policy and provides input into specific IPC issues. Simply stated, its function is to prevent and control healthcare-associated infections. That is accomplished in a variety of ways, some of which include: surveillance of infections, product evaluation, investigation of infection outbreaks and development of IPC procedures.

Kay, N. (2019). Communication skills: For nursing and healthcare students. Lantern Publishing.
Introduction
Effective communication is key to patient safety to ensure no harm occurs in the delivery of patient care. Nurses constitute the largest workforce in the NHS and play a vital role in ensuring patient safety, spending the most time with patients and performing many roles. These include providing effective and safe care, ongoing patient monitoring and coordination of care. Protecting patients from harm can be seen as fundamental in all nursing activities. Generally, the vast majority of patients accessing healthcare services will have a positive experience due to the high-quality, safe care delivered by dedicated healthcare professionals. However, errors and omissions in their care result in harm to some patients and most of these incidents are preventable. In a review of root cause analyses, communication was found to be an important causal factor (World Health Organization, 2008) and is one of the most common causes of dissatisfaction within healthcare services (Royal College of Nursing, 2017). Communication strategies are indicated in varying forms, as policies and procedures, performance statistics, incident reports, workplace inductions, learning from errors, education and training. These strategies are essential to engage healthcare professionals in patient safety activities that promote a positive safety culture. This chapter will provide an overview of patient safety and safety culture, and how human factor principles are applied to patient safety. The importance of learning from errors cannot be underestimated as this is key to promoting a positive safety culture. This relies, however, on effective incident reporting structures and strategies to support this process.
Concept of patient safety
At its simplest, patient safety is defined as the “prevention of patient harm” (Kohn et al ., 2000). The World Health Organization (2009, p. 15) offers a similar but broader definition and states that: “Patient safety is the reduction of risk of unnecessary harm associated with healthcare to an acceptable minimum.”
The use of the word ‘unnecessary’ in this definition recognises that errors, violation, patient abuse and deliberate unsafe acts (termed ‘incidents’) occur in healthcare. To standardise the terms used relating to incidents, the World Health Organization (2009) has published an International Classification for Patient Safety (ICPS). This conceptual framework was developed to enable categorisation of patient safety information by using a standardised set of concepts and agreed definitions. The table below illustrates some examples of the key concepts and definitions commonly used in clinical practice.
| Concept | Definition |
|---|---|
| Event | Something that happened to or involved a patient |
| Healthcare-associated harm | Harm arising from or associated with plans or actions taken during the provision of healthcare, rather than an underlying disease or injury |
| Patient safety incident | An even or circumstance that could have resulted, or did result, in unnecessary harm to a patient; these arise from violation or error |
| Violation | Intended acts due to deliberate deviation from a procedure or standard or rule |
| Error | Unintentional acts due to failure to carry out planned action as intended or application of an incorrect plan. Errors may manifest by doing the wrong thing (commission) or by failing to do the right thing (omission) at either the planning or execution phase |
| Harm | Implies impairment of structure or function of the body and/or damaging effect arising therefrom, including disease, injury, suffering, disability and death, and may be physical, social or psychological |
While healthcare brings enormous benefits to those using the health service, errors are common, and patients are frequently harmed. It is important to recognise that healthcare and healthcare delivery is a multifaceted phenomenon reflected by the complexities of health, social, political and organisational context (Curry and Nunez-Smith, 2015). Individual beliefs, values and motivations that underlie individual behaviours are also complex, and therefore keeping patients safe from harm is a significant issue and one of the most prominent healthcare challenges worldwide.
Safety culture
‘Safety culture’ is integral to the overall culture of an organisation. The following definition of safety culture was originally cited in the UK Health and Safety Commission report in 1993 and is quoted widely in the healthcare literature and many governments report:
The safety culture of an organisation is the product of the individual and group values, attitudes, competencies and patterns of behaviour that determine the commitment to, and the style and proficiency of, an organisation’s health and safety programmes…
(Health Foundation, 2013, p. 5)
Safety culture as a concept is usually described in terms of perceptions relating to trust, values and attitudes that focus upon preventing errors and maintaining patient safety. It also refers to the way in which patient safety is thought about, how this is implemented within an organisation, and the structures and processes in place to support this. All these factors have a huge positive or negative influence on patient safety outcomes. The effect of a healthcare organisation’s safety culture on patient clinical outcomes has been studied extensively, with communication identified as an important organisational aspect affecting patient safety (Wang et al ., 2014). A positive safety culture is therefore characterised by effective communication founded on mutual trust to keep patients safe from actual and potential harm. In comparison, a negative safety culture can cause harm and injury and can be characterised by poor communication of patient safety issues within clinical environments and across the organisations. The table below provides further characteristics that you may experience in your clinical practice areas.
| Positive safety culture | Negative safety culture |
|---|---|
|
|
In general, the overall concept of safety culture focuses upon preventing errors and maintaining patient safety, which may seem straightforward. However, promoting a positive safety culture is multifaceted due to the different dimensions that are associated with it. The Agency for Healthcare Research and Quality (AHRQ) identified 12 dimensions when it developed the Hospital Survey on Patient Safety Culture (HSOPSC). This is a pre-validated questionnaire that is widely used internationally to study and evaluate individual perceptions of safety culture in hospital settings (AHRQ, 2012). Other pre-validated questionnaires such as the Safety Attitude Questionnaire (SAQ) (developed by Sexton et al ., 2006) also identify similar dimensions. It is beyond the remit of this chapter to discuss safety culture questionnaires in detail. Further information can be found on The Health Foundation website. This document provides a summary of the surveys, together with their strengths and weaknesses.
Incident reporting
Since the publication of An Organisation with a Memory (Department of Health, 2000), healthcare organisations have made significant efforts to reduce patient safety incidents and subsequent harm. It is now recognised that an organisation’s safety culture and approach to patient safety incidents are key factors influencing safety and quality. As a form of communication, incident reporting is an established mechanism for improving patient safety. Organisations with a positive safety culture (as illustrated in Table 8.3 ) report a large number of patient safety incidents and acknowledge healthcare professionals for their candour and commitment to learning. In comparison, those organisations with a negative safety culture will blame individuals and consider reporting those incidents ‘out of line’ (Taylor, 2012). It is important to note that an increased number of reported patient safety incidents reflects an improved reporting culture and should not be interpreted as a decrease in the safety of the NHS. Equally, a decrease cannot be interpreted as an increase in the safety of the NHS. Evidence suggests that there is a positive correlation between safety incident reporting data and a high Hospital Mortality Ratio Score (HMRS) for those organisations that report large numbers of incidents (Keogh, 2013). Conversely, it is recognised that only a relatively small percentage of incidents that occur are actually reported. In addition, those incidents that are considered ‘near misses’ were not reported. In nursing, the reasons for failure to report incidents have been extensively researched (for example, Alahmadi, 2010; El-Jardali et al ., 2014) and findings reveal a number of barriers to reporting, for example:
- time constraints
- failure to recognise an incident.
- feeling threatened
- fear of blame
- failure to receive feedback.
Any patient safety incident is a reportable circumstance where there is significant potential for harm, near miss or no harm (adverse event) for one or more patients receiving healthcare. Another key challenge when reporting a patient safety incident is the terminology that is used within the reporting systems. As a student, you will encounter unintended or unexpected patient safety incidents in your placement experiences and it is fundamental that you “demonstrate an understanding of how to identify, report and critically reflect on near misses, critical incidents, major incidents and serious adverse events in order to learn from them and influence their future practice” (NMC, 2018b, p. 22). The cycle image demonstrates the process that should be followed when patient safety incidents occur.
Summary
Nurses constitute the largest workforce in healthcare. They spend most time with patients and therefore play a vital role in patient safety. To improve patient safety, a positive safety culture must exist to protect patients from unnecessary harm. Safety culture is a multifaceted concept that incorporates many dimensions, but the fundamental element is communication, which has been discussed in this chapter. Understanding how harm does occur to patients, and reporting of errors so that lessons can be learnt, are vital to improving patient safety. A number of strategies and tools can be used to encourage learning from errors and more than one approach can be used. This chapter has focused on the use of patient stories as a method of learning from ‘real life’ clinical incidents and it is hoped that you can adopt this method to share with colleagues, peers and the wider healthcare teams.
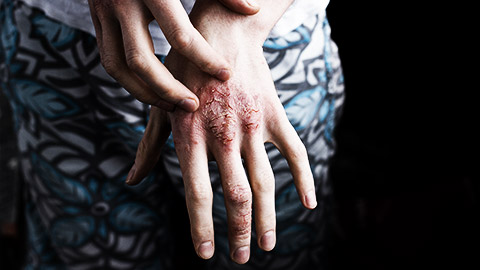
Aydin, A. I., Atak, M., Nurcan, O. N., & Dalkizan, V. (2021). Hand dermatitis among nurses during the COVID-19 pandemic: Frequency and Factors. WCET Journal, 41(4), 10-14. https://doi.org/10.33235/wcet.41.4.10-14
Introduction
The World Health Organisation has declared COVID-19 a global pandemic. According to the latest data, approximately 230 million people have been infected and 4.7 million have died. Nonpharmacologic preventive practices such as maintaining social distance, wearing face masks, and washing hands have been recommended to hinder the spread of the virus. These practices play an important role in reducing the risk of transmission by preventing the spread of aerosols and protecting vulnerable populations. Studies suggest that hand hygiene is an important prevention strategy for healthcare professionals and societies in places where the pandemic is most prevalent. Hand hygiene remains a critical element of infection control.
The COVID-19 pandemic has also had the effect of reminding nurses about the need for regular hand hygiene with soap, water, or alcohol-based sanitisers. Handwashing is recommended as an inexpensive and common preventive measure to protect oneself from a number of viral infections and prevent disease spread in general. Proper handwashing technique is a part of effective hand hygiene. The CDC recommends handwashing with soap because it reduces the amount of germs and chemicals on the hands. The World Health Organisation (WHO) also recommends handwashing with soap for 40 to 60 seconds using an appropriate technique when hands are noticeably dirty. When soap and water are not available, a hand sanitiser with at least 60% alcohol can be used. If hands are not visibly soiled, using an alcohol-based hand disinfectant for 20 to 30 seconds with the appropriate technique is preferred to provide hand hygiene. Washing hands with an alcohol solution can reduce the risk of infection in medical staff and others in the community by reducing the number of bacteria and viruses on hands.
However, the solutions used, frequency of handwashing, level of moisture, and hand drying process may disrupt the skin barrier and lead to symptoms of hand dermatitis. Accordingly, the aim of this study was to determine the frequency of hand dermatitis among nurses during the COVID-19 pandemic and the factors affecting its incidence.
Nurse’s hand hygiene
The frequency of hand dermatitis among the nurses was 70.9% of all nurses that were questioned. The frequency of hand dermatitis was significantly higher in women than in men. The frequency of hand dermatitis was significantly higher among nurses who had a history of allergies compared with those without an allergy. No significant difference was found in the frequency of hand dermatitis among nurses by mean age or years of employment. When the incidence of hand dermatitis was examined by unit where the nurses worked, the frequency was higher among nurses working in pediatric (76.1%), surgical (76%), and COVID-19 wards (69.5%). However, no significant difference was found by unit. The most frequently reported symptoms were redness and fissures (sharply defined linear tears in the epidermis and dermis; 77.1%), irritation and itching (76%), and scaling/rash (67.4%).
The frequency of hand dermatitis was 71.5% among nurses who provided care to patients who were COVID-19 positive, whereas the frequency of hand dermatitis was 66.7% among nurses who did not provide care to COVID-19-positive patients. This difference was not significant. Nurses’ hand hygiene behaviours before and during the COVID-19 pandemic were examined. It was determined that the frequency of handwashing per day, the use of disinfectants, and the use of hand cream/moisturiser had increased significantly during the pandemic. In terms of handwashing frequency, 48% (84 of 175) of the nurses washed their hands more than 25 times a day. The frequency of hand dermatitis significantly increased with the frequency of handwashing. The frequency of hand disinfectant and hand cream use did not significantly affect hand dermatitis. The most frequently used handwashing substances were liquid soap, liquid soap and alcohol-based gel, chlorhexidine-based gel, and alcohol-based gel.
Discussion
Hand dermatitis is a common disease that can progress either acutely or chronically and has different etiologies. In the current study, the frequency of hand dermatitis among nurses during the COVID-19 pandemic was 70.9%. The results of the current study were similar to those obtained in other studies conducted on hand dermatitis in health workers during the COVID-19 pandemic. One study conducted during the pandemic revealed that 84.6% of health workers had unwanted skin reactions on their hands. Another study reported that 74.5% of primary health workers had skin damage on their hands. A study conducted at the beginning of the pandemic found that 90.4% of health workers had acute symptoms of hand dermatitis.
In a study conducted before the pandemic in the same region as the current study, 47.5% of the nurses working in pediatric clinics were reported to have hand dermatitis. The frequency of hand dermatitis was found to be 12%, 21%, and 22.1% in other studies conducted before the pandemic. Given these results, it is clear that the frequency of hand dermatitis had increased among health workers during the pandemic.
In this study, the frequency of hand dermatitis was significantly higher in women than in men. Alluhayyan et a conducted a study with health workers and found that women were more prone to dermatitis. Likewise, Gupta et al found that hand dermatitis was slightly more common in women than in men. However, other studies have reported that sex did not have a significant effect on the frequency of hand dermatitis.
Allergies are abnormal hypersensitivity reactions of the immune system against foreign substances. This response can be observed in early childhood as well as in adolescence and adulthood. The frequency of hand dermatitis was significantly higher among nurses who had a history of allergy compared with those without an allergy, which is consistent with previous studies. However, Kiely et al27 concluded that a history of allergy did not affect the development of dermatitis, despite reporting that the risk of developing dermatitis was significantly higher in health workers with a history of dermatitis.
One of the most common methods to prevent the spread of viruses is effective hand hygiene. In the fight against COVID-19 it is essential that effective hand hygiene habits are acquired in childhood. Kiely et al reported that the frequency of handwashing increased among almost all health workers (99.26%) during the pandemic. When the pre-pandemic and pandemic periods were compared in the current study, the frequency of handwashing and use of hand disinfectants/creams had increased significantly (Table 3). Similarly, Guertler et al conducted a study with physicians and nurses and found similar results. All of the guidelines for combating COVID-19 recommend thorough and frequent hand hygiene practices. Although the increase in the frequency of handwashing is one of the factors that contributes to the development of dermatitis, this should not dissuade health workers from appropriate hand hygiene practices during the pandemic.
Nurses are the primary caregivers in a medical setting and are thus prone to infection with and transmission of the COVID-19 virus. It is vital that they comply with the guidelines for preventing and controlling infections to fight the pandemic. In another study conducted during the pandemic, Lan et al concluded that the frequency of hand dermatitis increased significantly in health workers who washed their hands more than 10 times a day. Studies conducted before the pandemic reported a significant relationship between an increased frequency of handwashing and the frequency of hand dermatitis. However, the present study found no significant difference in the frequency of hand dermatitis by use of hand cream. However, the hand creams used by nurses may not be ideal. The use of skin moisturisers is recommended to maintain healthy skin; for skin protection, humectants such as topical urea and propylene glycol and occlusive emollients such as petrolatum-based products, lanolin, mineral and vegetable oils, and waxes together are recommended. Concomitant use is beneficial to keep the stratum corneum moist and soothe the skin.33
Conclusions
This study found that the frequency of hand dermatitis among nurses during the pandemic was high. Sex, history of allergy, and increased frequency of handwashing were among the factors increasing hand dermatitis. Hand hygiene increased significantly among health workers during the pandemic. It was also found that nurses increased the measures they took to protect their skin to avoid dermatitis. Hand disinfection plays an important strategic role in the fight against COVID-19. However, the skin and mucosa barrier are likely to be damaged in nurses who are consistently practicing good hygiene. Nurses should take proper measures to protect their skin while carrying out their duties.

Ambigapathy, S., Rajahram, G. S., Shamsudin, U. K., Khoo, E. M., Cheah, W. K., Peariasamy, K. M., Goh, P. P., & Khor, S. K. (2020). How should front-line general practitioners use personal protective equipment (PPE)? Malaysian Family Physicians, 15(1), 2-5.
Introduction
The COVID-19 outbreak continues to evolve, and there is a possibility that larger-scale community outbreaks could occur across Malaysia, placing a significant burden on general practitioners (GPs) to assess suspected cases. However, as the risk associated with COVID-19 infection continues to evolve, GPs must act consistently with updated guidance on the appropriate use of personal protective equipment (PPE) such as masks, gloves, gowns and eye protectors. This commentary focuses on the appropriate use of PPE for front line GPs to complement official guidance on its use.
PPE is only one part of risk mitigation for GPs.
In GP clinics, a hierarchy of control measures should be used to mitigate risk of infectious diseases. PPE is an important part of a basket of solutions and should be considered as supplementing but not substituting other measures such as administrative, environmental and engineering controls. Administrative controls include ensuring appropriate infrastructure, clear infection prevention and control policies, facilitated access to laboratory testing, appropriate triage and placement of patients, and adequate staff-to-patient ratios.
In parallel, environmental and engineering controls reduce contamination of surfaces and inanimate objects, and hence the spread of pathogens. Where possible, clinics must provide adequate space of at least 1 meter to be maintained between all persons, and ensure that well-ventilated isolation rooms are available for patients with suspected or confirmed disease. The use of PPE may be seen as cumbersome, nonetheless, GPs must choose the right type of PPE, and be knowledgeable in wearing, removing and disposing used PPE. However, in an outbreak, PPE alone is not a magic solution, and other measures including good hand hygiene and social distancing should be prioritised.
How should GPs receive information about PPE
GPs must perform risk assessments to determine the most suitable combination of PPEs for their individual clinics. As the situation evolves, GPs need to be aware of and adhere to the latest updated guidelines on the use of PPE from the COVID-19 Management Guideline by Ministry of Health Malaysia (currently Version 4.0). There are several types of PPEs manufactured with different standards and methods for donning, removing and disposal of the PPE. It is advisable for GPs to follow the manufacturers' recommendations and complement it with the recommendations from the MOH.
Other sources of information for PPE and PPE quality assurance standards come from the Standards & Industrial Research Institute of Malaysia (SIRIM) and the Department of Occupational Safety and Health (DOSH) under the Ministry of Human Resources.
How should GPs set-up their clinics and use PPE?
At the entrance of the clinic, clear signage such as posters and visual alerts in local languages should be placed to inform patients who fall under the category of patients under investigation (PUI) and ensure that they notify the health personnel at triage counters or receptions. PUI are defined as patients who have fever or acute respiratory infection (sudden onset with at least one of the following: shortness of breath, cough or sore throat) and have travelled to or reside in affected countries in the 14 days prior to illness, or have close contact with a confirmed case of COVID-19 in the 14 days before onset of illness. In addition, healthcare personnel at triage counters or receptions need to undertake risk assessment of all patients and visitors to identify possible PUI. This risk assessment is based on the MOH guidelines. Healthcare personnel should wear a face mask and regularly use an antiseptic hand rub or alcohol-based hand sanitizer at the counter.
Once a PUI is identified, they must be placed in a special isolation room (where available) or designated waiting area. This area should be well ventilated allowing staff and other patients to be placed 1 meter apart, free of clutter and with minimal fixtures. It should be equipped with a no-touch bin to discard used tissue and hand sanitizer dispensers.
In most instances, a physical examination is not required prior to referral to a designated hospital. However, if a physical examination for a PUI is warranted, healthcare personnel must wear N95 masks (fit checked) or surgical masks with face shield or goggle, standard isolation gown (fluid repellent long-sleeved gown) and gloves. There should be strict adherence to frequent and strict hand hygiene when examining patients in the isolation room. All healthcare personnel must be skilled in the process of donning and doffing PPE. A video link to these procedures can be found on the official social media page of the Director-General of Health of Malaysia.
After examining patients, cleaning and disinfection according to standard procedures must be followed. Waste management, packing and transporting patient-care equipment, linen and laundry must be performed according to standard infection control procedures as described by the Department of Environment, Malaysia. Moreover, to avoid physical interaction with suspected COVID-19 cases, clinics can consider rescheduling routine appointments or ensure appropriate measures are taken to isolate high-risk patients.
Triaging and referrals
All PUI should be offered hand sanitizer and surgical masks, provided the patient is not tachypnoeic or hypoxic. If the patient is unable to tolerate these, the patient is advised to cover their nose and mouth during coughing and sneezing with tissue. Patients, especially foreigners, must be asked for their Health Alert Cards, which are given at the point of entry into Malaysia if they have travelled from affected countries. All PUIs should be referred to the nearest MOH hospitals accepting patients. This list is regularly updated on the MOH website and the COVID-19 Management Guidelines. Each PUI must be discussed with the Infectious Disease (ID) Physician/Physician at the designated hospital before transfer. PUI must never be allowed go to MOH designated hospitals on any form of public transport or private hire vehicles. GPs can liaise with the local District Health Office or designated hospital emergency department to arrange transport for these patients. They must wear a face mask during the journey. The ID Physician or Physician will be able to guide the attending GPs further.
Educating patients about PPE: How and what?
Currently, there is no evidence that those without respiratory symptoms should wear face masks. If a patient has cough, they are advised to practise good cough etiquette, which includes covering the nose and mouth with tissue whenever coughing or sneezing, to throw the tissue into proper trash bins immediately after use and to wash their hands with soap and water or use hand sanitizer frequently. If a tissue is not available, they are advised to use the fold of their elbow. These patients are also advised to wear a face mask.
PUIs who do not fulfil criteria for admission to hospital will be placed under home surveillance and be monitored daily by the district health office for 14 days. During this time, they are strictly prohibited from leaving their home. Other measures prescribed are available in the home surveillance assessment tool.
GPs should also provide patient and family with ongoing support, education and monitoring. This can be done by using Health Alert Cards with information useful for patient and family and counselling on any concerns they may have. This Health Alert Card can be easily obtained from the COVID-19 Management Guidelines 2020 of Ministry of Health Malaysia. GPs can make photocopies of these cards to be given to patients who come to their clinics.
Rutala, E. A., & Weber, J. D. (2016). Disinfection and sterilisation in healthcare facilities. Infectious Disease Clinics, 30(3), 609-637. https://doi.org/10.1016/j.idc.2016.04.002
In the United States in 2010 there were approximately 51.4 million inpatient surgical procedures and an even larger number of invasive medical procedures. In 2009, there were more than 6.9 million gastrointestinal (GI) upper, 11.5 million GI lower, and 228,000 biliary endoscopies performed. Each of these procedures involves contact by a medical device or surgical instrument with patients’ sterile tissue or mucous membranes. A major risk of all such procedures is the introduction of pathogenic microbes, which can lead to infection. Failure to properly disinfect or sterilize equipment may lead to transmission via contaminated medical and surgical devices (e.g., carbapenem-resistant Enterobacteriaceae [CRE]).
Achieving disinfection and sterilization through the use of disinfectants and sterilization practices is essential for ensuring that medical and surgical instruments do not transmit infectious pathogens to patients. Because it is not necessary to sterilize all patient-care items, health care policies must identify whether cleaning, disinfection, or sterilization is indicated based primarily on each item’s intended use, manufacturers recommendations, and guidelines. Multiple studies in many countries have documented lack of compliance with established guidelines for disinfection and sterilization.
Failure to comply with scientifically based guidelines has led to numerous outbreaks and patient exposures. Because of noncompliance with recommended reprocessing procedures, the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) issued a health advisory alerting health care providers and facilities about the public health need to properly maintain, clean, and disinfect and sterilize reusable medical devices in September 2015. In this article, which is an updated and modified version of earlier articles, a pragmatic approach to the judicious selection and proper use of disinfection and sterilization processes is presented, based on well-designed studies assessing the efficacy (via laboratory investigations) and effectiveness (via clinical studies) of disinfection and sterilization procedures.
A rational approach to disinfection and sterilisation
Almost 50 years ago, Earle H. Spaulding devised a rational approach to disinfection and sterilization of patient-care items or equipment. This classification scheme is so clear and logical that it has been retained, refined, and successfully used by infection control professionals and others when planning methods for disinfection or sterilisation.
Spaulding thought that the nature of disinfection could be understood more readily if instruments and items for patient care were divided into 3 categories based on the degree of risk of infection involved in the use of the items. The 3 categories he described were critical, semi critical, and noncritical. This terminology is used by the CDC’s “Guidelines for Environmental Infection Control in Healthcare Facilities” and the CDC’s “Guideline for Disinfection and Sterilization in Healthcare Facilities.”
Critical items
Critical items are so called because of the high risk of infection if such an item is contaminated with any microorganism, including bacterial spores. Thus, it is critical that objects that enter sterile tissue or the vascular system be sterile because any microbial contamination could result in disease transmission. This category includes surgical instruments, cardiac and urinary catheters, and implants used in sterile body cavities. The items in this category should be purchased as sterile or be sterilized by steam sterilization if possible. If heat sensitive, the object may be treated with ethylene oxide (ETO), hydrogen peroxide (HP) gas plasma, vaporized HP, HP vapor (HPV) plus ozone, or by liquid chemical sterilant if other methods are unsuitable.
Liquid chemical sterilant can be relied on to produce sterility only if cleaning, which eliminates organic and inorganic material, precedes treatment and if proper guidelines as to concentration, contact time, temperature, and pH are met. Another limitation to sterilization of devices with liquid chemical sterilant is that the devices cannot be wrapped during processing in a liquid chemical sterilant; thus, it is impossible to maintain sterility following processing and during storage. Furthermore, devices may require rinsing following exposure to the liquid chemical sterilant with water that, in general, is not sterile. Therefore, because of the inherent limitations of using liquid chemical sterilant in a nonautomated (or automated) reprocessor, their use should be restricted to reprocessing critical devices that are heat sensitive and incompatible with other sterilization methods.
Summary of advantages and disadvantages of commonly used sterilisation techniques
| Sterlisationa Method | Advantages | Disadvantages |
|---|---|---|
| Steam |
|
|
| HP Gas Plasma |
|
|
| 100% ETO |
|
|
| Vapourised HP |
|
|
Semi-critical items
Semi critical items are those that come in contact with mucous membranes or nonintact skin. Respiratory therapy and anaesthesia equipment, gastrointestinal endoscopes, bronchoscopes, laryngoscopes, Endo cavitary probes, prostate biopsy probes,28 cystoscopes, hysteroscopes, infrared coagulation devices, and diaphragm fitting rings are included in this category. These medical devices should be free of all microorganisms (i.e., mycobacteria, fungi, viruses, bacteria), although small numbers of bacterial spores may be present. Intact mucous membranes, such as those of the lungs or the gastrointestinal tract, are generally resistant to infection by common bacterial spores but susceptible to other organisms, such as bacteria, mycobacteria, and viruses.
Because semi critical equipment has been associated with reprocessing errors that result in patient lookback and patient notifications, it is essential that control measures be instituted to prevent patient exposures. Before new equipment (especially semi critical equipment as the margin of safety is less than that for sterilization) is used for patient care on more than one patient, reprocessing procedures for that equipment should be developed. Staff should receive training on the safe use and reprocessing of the equipment and be competency tested. At the University of North Carolina (UNC) Hospitals, to ensure patient-safe instruments, all staff that reprocess semi critical instruments (e.g., instruments which contact a mucous membrane such as vaginal probes, endoscopes, prostate probes) are required to attend a 3-hour class on HLD of semi critical instruments. The class includes the rationale for and importance of high-level disinfection, discussion of high-level disinfectants and exposure times, reprocessing steps, monitoring minimum effective concentration, personal protective equipment, and the reprocessing environment (establish dirty-to-clean flow). Infection control rounds or audits should be conducted annually in all clinical areas that reprocess critical and semi critical devices to ensure adherence to the reprocessing standards and policies. Results of infection control rounds should be provided to the unit managers, and deficiencies in reprocessing should be corrected and the corrective measures documented to infection control within 2 weeks (immediately correct patient safety issues, such as exposure time to high-level disinfectant).
Non-critical items
Noncritical items are those that come in contact with intact skin but not mucous membranes. Intact skin acts as an effective barrier to most microorganisms; therefore, the sterility of items coming in contact with intact skin is “not critical.” Examples of noncritical items are bedpans, blood pressure cuffs, crutches, bed rails, linens, bedside tables, patient furniture, and floors. In contrast to critical and some semi critical items, most noncritical reusable items may be decontaminated where they are used and do not need to be transported to a central processing area. There is virtually no documented risk of transmitting infectious agents to patients via noncritical items when they are used as noncritical items and do not contact nonintact skin and/or mucous membranes. However, these items (e.g., bedside tables, bed rails) could potentially contribute to secondary transmission by contaminating hands of healthcare personnel or by contact with medical equipment that will subsequently come in contact with patients. The table below list several low-level disinfectants that may be used for noncritical items. The exposure time for low-level disinfection of noncritical items is at least 1 minute.
| Disinfectant | Active Advantages | Disadvantages |
|---|---|---|
| Alcohol |
|
|
| Sodium hypochlorite |
|
|
Summary
When properly used, disinfection and sterilization can ensure the safe use of invasive and non-invasive medical devices. The method of disinfection and sterilization depends on the intended use of the medical device: critical items (contact sterile tissue) must be sterilized before use; semi critical items (contact mucous membranes or nonintact skin) must be high-level disinfected; and noncritical items (contact intact skin) should receive low-level disinfection. Cleaning should always precede HLD and sterilization. Current disinfection and sterilization guidelines must be strictly followed.
Conserve. (n.d.) The hierarchy of control & WHS. https://www.conserve.com.au/blog/the-hierarchy-of-control-amp-whs
Safework New South Wales. (n.d.). Control measures. https://www.safework.nsw.gov.au/__data/assets/pdf_file/0006/446028/hierarchy-of-controls-SW09182.pdf
When an employee is injured in the workplace, the next step is to review the events leading up to the incident—after any medical assistance, of course! The outcome of the review will often result in:
- Tighter controls
- Better training
- More PPE
This is great, but wouldn’t it be better if the risk could be eliminated entirely? Well, that’s where the Hierarchy of Control comes in. When considering the causes and outcomes of a workplace incident—or risk identified by a risk assessment—the best course of action is to work through a series of possible risk control measures. Because what’s better than simply reducing the chance of risk? Eliminating it completely.
What is the hierarchy of control anyway?
The Hierarchy of Control pyramid is a sequence of steps that should be considered when evaluating ways to remove or reduce a discovered risk. Think of it like the food pyramid—each part is important, but preference should be given to the control measures that are bigger in the hierarchical structure than those that are smaller. Or, more simply when you look at the following image, start from top to bottom!
Of course, the most effective risk control will often come from simultaneously implementing a number of levels from the hierarchy.
What are the risk control measures, and where does each one fit into the hierarchy?
Eliminating the Risk (Level One)
Our highest level is complete risk elimination, and it is always the preferred option when available as it means there is zero chance of the incident reoccurring. While removing the risk altogether may not be possible, it should always be the first control measure explored (in most cases). An example of risk elimination could be providing extending poles operated from the ground to access a high window latch rather than climbing a ladder, which presents a risk of falling.
Substituting the Risk (Level Two)
The next level down in the hierarchy is risk substitution. Risk substitution is the process of removing risk by replacing it with another that is either less likely to occur or less severe in its potential damages. Substitution is less preferred as it still leaves a risk present, even in a reduced form. An example of risk substitution could be replacing noisy equipment with a quieter option or replacing a highly toxic chemical with a less dangerous version. After the substitution is complete, it’s important to conduct a new risk assessment to identify any new risks created by the substitution process.
Isolate the Risk (Level Three)
The third level in the hierarchy is risk isolation. Risk isolation is performed by placing some form of barrier between the employee and the risk factor to provide protection. The key difference between this level and risk elimination (level one) is that the risk is still present, but a barrier shields the employee. If the barrier were to fail or require bypass, the risk would return to being uncontrolled. Risk isolation could be enacted by placing dangerous machinery in a separate room from the operating and installing remote control systems.
Engineering Controls (Level Four)
Engineering risk control is the process of designing and installing additional safety features to workplace equipment. Safety features could be installing more stringent ventilation systems in toxic environments or installing guardrails on a raised walkway.
Administrative Controls (Level Five)
Level five of the hierarchy is administrative controls. These are measures the management and chain-of-command can implement to reduce the likelihood of a risk occurring. Measures could include providing dedicated training targeted at the risk or arranging work schedules to limit exposure times in hazardous environments.
Personal Protective Equipment (Level Six)
The final level in the hierarchy of risk control is the use of PPE. This level will likely be utilised regardless of what other levels are also being used to control risk. However, it remains at the bottom of the hierarchy as it doesn’t remove or reduce the risk itself. Instead, this level is designed to assume an incident will occur and protect the employee from harm when/if it does. PPE includes items such as hard hats, noise-reducing ear protection, cut-resistant gloves, and more.
How does the hierarchy increase workplace safety?
The risk control measures are implemented in order of their effectiveness at controlling the risk and preventing accidents or injury. Risk elimination sits at the top of the hierarchy as there is no better way to prevent accidents or injuries than completely removing the risk. Following down the hierarchy, isolating a risk completely, for example, by moving a loud and vibrating machine into a room to be controlled remotely, is better than just installing some soundproofing (engineering) or limiting employee exposure times (administrative). PPE is a somewhat unique control on the hierarchy, as it is both the least effective (sitting at the bottom) and most commonly used. That is because PPE still leaves the risk uncontrolled — the employee is only personally protected from injury when the risk causes an incident.
PPE is unique, though, as it will usually be used in conjunction with whatever other risk control measures have been implemented. It acts as a final line of defence. It is also easy and cheap to implement compared to engineering completely new machinery or safeguards, making it perfect to use in addition to other controls. It also offers the additional benefit of protecting the worker from injury due to undiscovered risks.
It is good practice to self-enforce the hierarchy. In some jurisdictions, it is also a legal requirement to attempt to eliminate risks before attempting any other control measures anyway, so why not get ahead from the outset?
Always remember: safety first!
The image below from Safe Work Australia, depicts the right controls to eliminate or minimise risks and to protect your workers.
Control measures

