In this lesson we continue to look at dispensing legislation and regulations.
Medicines legislation
As a pharmacy technician, your work will include handling prescription medicines, over-the-counter medicines, and related products. To ensure patient health and safety, it is important for you to comply with the relevant medicines legislation about the handling of these products. To learn about this, read Part 5, Sections 26-37 of the Medicines Regulations 1984 and answer these questions.
Answer the following six questions about medicines legislation.
Under the Medicines Regulations, certain medicines that are particularly hazardous require Safety Caps or Child Resistant Closures. These caps are designed to make it more difficult for children to access the contents of the medicine and accidentally poison themselves. However, it's important to note that these caps are not completely childproof and should not be relied on as a substitute for safe storage practices.
The medicines that require Safety Caps include:
- aspirin and its salts (as well as medicines containing it)
- iron-containing more than 24mg of elemental iron
- paracetamol (and medicines containing it)
- classes of central nervous system depressants
- classes of antipsychotics and antidepressants.
Misuse of Drugs Legislation
The Misuse of Drugs legislation requires pharmacies to maintain accurate and up-to-date records of controlled drugs to minimise the risk of harm to patients and the public.
To achieve this, pharmacies are required to keep a Controlled Drugs Register. Every time a CD is dispensed or disposed of and every time a CD is received by the pharmacy from the supplier, it must be recorded in the register.
The records must contain the following information:
- The date
- The surname, initials and address of the person supplied
- Address of the CD supplier (wholesaler or manufacturer)
- Prescription or order number
- Quantity dispensed or quantity received from the supplier
- A running balance
- Surname, initials, and address of the prescriber
- Signature of the person dispensing and initials of the person checking

Search for the answers to these questions about the Controlled Drugs Register in Part 6 of the Misuse of Drugs Regulations 1977.
Instructions:
Read the following four questions and make notes on your responses.
Questions:
- What is the form that a Controlled Drug Register must take as described by the regulation?
- Who must keep a Controlled Drug Register?
- Where must pharmacies keep the Controlled Drug Register, and in what ‘manner’ as described in the regulation?
- How long must the Controlled Drug Register be kept for?
CDs returned to the pharmacy
Controlled drugs that are returned to the pharmacy because they have not been used or have expired must also be entered in the Controlled Drugs Register. This must be on a separate page, often a page in the back of the register. This is where all drugs to be disposed of will be written up. Two pharmacists or a pharmacist and a responsible person, such as a doctor, may then destroy them. They must be dated and signed out of the register by both people. A record of destruction may (this is not compulsory) be sent to the local Medicines Control Advisor.
For each controlled drug destroyed, the record of destruction must give the reason for destruction and the following:
- The name and address of the pharmacy
- The name of the drug
- The strength
- The dose form
- The quantity
Use the navigation below the image to progress through each of the slides to see an example of a controlled drug register.
Another important legal requirement related to the strict controls on CDs is described in Part 6 of the Misuse of Drugs Regulations 1977. This regulation outlines the requirements for stocktaking.
Instructions:
Read the following two questions and make notes on your responses.
Questions:
- Who does this regulation apply to?
- What are the requirements set out in this regulation — what must be done?
Secure storage
Pharmacists and other people responsible for the possession of CDs for supply must ensure that they are stored securely. This is to prevent the loss or theft of medicines that have the potential to cause serious harm.
|
Part 4 of the Misuse of Drugs Regulation outlines how CDs not for immediate use must be stored. This is a typical storage system from Kiwi company ACCESS Lock Specialists Ltd. |
Source: ACCESS Lock Specialists |
Health Practitioners Competence Assurance Act 2003 (HPCA Act)
For this activity, you’ll need to carry out your own research and answer questions about the information you learned.
You will find these links helpful:
- Read: Health Practitioners Competence Assurance Act 2003: NZ Legislation
- Read: Health Practitioners Competence Assurance Act: Ministry of Health
- Read: About the act
- Read: Responsible authorities
- Read: Professional and regulatory bodies: Ministry of Health
- Watch (2.30): How Health Regulators Work to Protect the Health of all New Zealanders: OT board of NZ
- Read: Pharmacy Council: What we do
Instructions:
Read the following nine questions and make notes on your responses.
Questions:
- What is the purpose of the Health Practitioners Competence Act?
- Under Part 1, Section 5 of the Act, what is the definition of the following:
- Health Practitioner
- Authority
- What is the punishment for a person who commits an offence against the Act by falsely claiming to be a health practitioner?
- Explain what is meant by ‘responsible authorities’?
- According to the Ministry of Health, how many professions are regulated by a responsible authority under the HPCA Act?
- Who is the responsible authority for the following professions:
- Nursing
- Medicine
- Midwifery
- Chiropractic
- Who is the responsible authority for the pharmacy profession
- Give both the English name
- Give the Māori name and its translation
- Describe what this authority does
- Under the HPCA, pharmacists must be registered with the relevant responsible authority and also hold what current document in order to be legally allowed to practice?
- Under section 64 of the HPCA, what must a responsible authority do if they receive a complaint about a health practitioner?
By this point in your learning, you will understand the term ‘scope of practice’ and what it means to work within the scope of your practice. Fill in the blanks in this paragraph from the Ministry of Health’s webpage about responsible authorities under the HPCA Act.
Self-directed learning activities:

This poster is for sale in Australia to remind staff that they need to be vigilant about safety around the disposal of sharps.
Task 1:
Refer to Part 4 of Misuse of Drugs Regulation, section 28, to create your own graphic of the legal requirements of how controlled drugs should be stored in a pharmacy.
Your graphic should contain pictures and words to describe the requirements. It can be made by hand (take a picture of it to upload) or digitally with any software you have available.
This is a learning activity, not an assessment. The purpose is to show your understanding of the legal requirements.
Task 2:
Reflect on how the HPCA Act applies to your daily work as a pharmacy technician. You might like to think about:
- What does ‘competent and fit to practice’ look like for a pharmacy technician?
- How can pharmacy technicians maintain their competence to practice over time?
Tau kē – you have completed another week of Professional Practice Part 1!

Service user subsidy categories
The codes that appear on a prescription are called Service User Subsidy Categories. They are made up of a letter and a number. They tell the pharmacy how much the customer will contribute towards their prescription. This is called a co-payment. You will be familiar with these codes from your previous learning. Take a moment to review the categories. Repetition will help you remember these codes so you can apply them to your work in the pharmacy.
| Y | Youth (0-13 years) |
| J | Junior (14-17 years) |
| A | Adult (over 18 years) |
| Z | HUHC Holder/Care Plus Service User |
| H | Hokianga resident enrolled with the HHET |
| O | Oral Contraceptive |
| X | Exempt. PSC Holder |
| 1 | CSC Holder |
| 3 | No CSC, and not Approved Prescriber |
| 4 | Approved Prescriber |
| NS | Not subsidised |
Challenge yourself to type in the correct answer in this next activity.
As of July 2023, there are legislative changes that have affected consumer co-payments.
Let's review the definition of a co-payment. Look through the A-D options below, come up with your answer, and then expand the label or (+) sign below to see if you are right.
A - A small charge towards the cost of paying for the pharmacist to do the final accuracy check on the prescription
B - A small charge towards the cost of paying for the bags that the pharmacy put a prescription in
C - A small charge towards the cost of prescribed medicines
D - A small charge used when the pharmacy is located in a leased space, such as inside a Countdown or Emergency Clinic.
C: A small charge the consumer pays towards the cost of prescribed medicines.
Review the changes for each user subsidy category by reading through this table: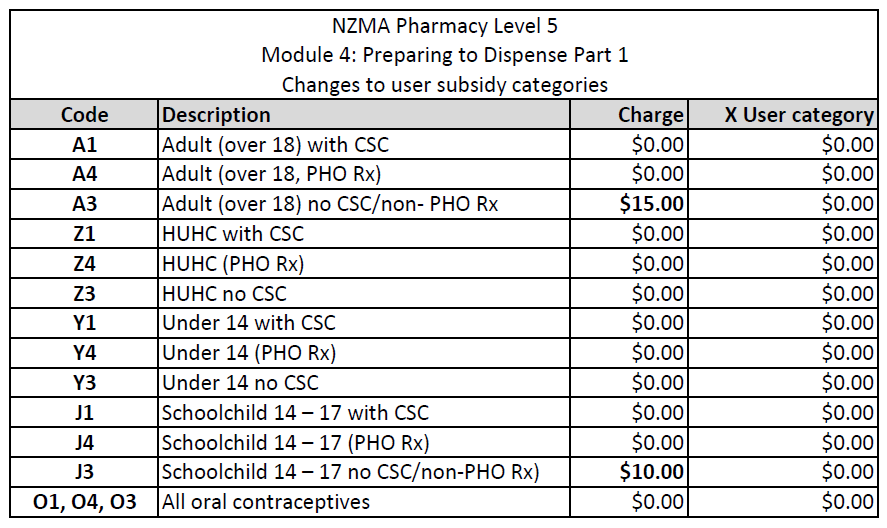
Download the table for your records and for future reference.
Take a look at these subsidy cards:
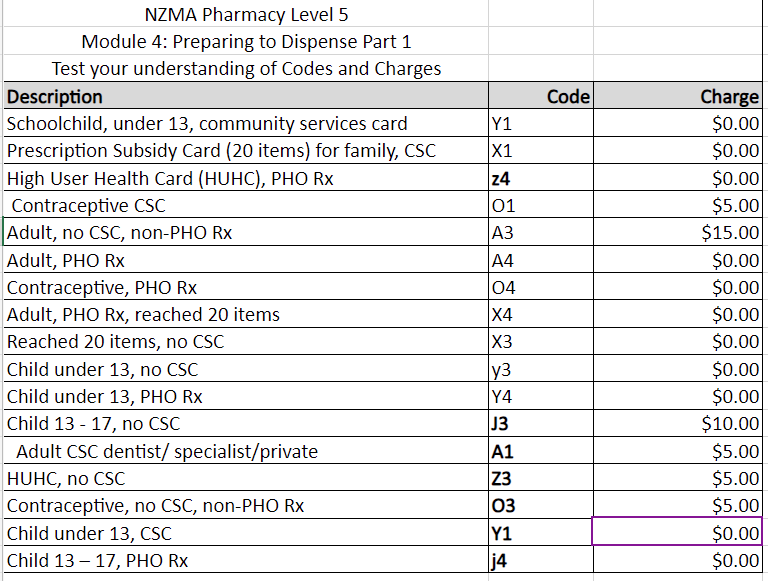
Ka pai for learning the subsidies and categories. This next activity will give you a chance to assign a code and charge.
- Download this Excel template with descriptions of patient subsidy information.
- Use the table above to complete the template on your own.
- Return here to check your work by comparing your table to ours. Expand the label by clicking it or the (+) sign to check your work.
No code?
If you receive a prescription that does not have a user subsidy category code, you can check if the customer is eligible for a subsidy by:
- checking with the prescriber
- asking the customer if they have a Community Services card, High Health User card, Prescription Subsidy card or SuperGold card
- look at the patient history in the computer system
- the pharmacist can decide what the correct payment is if they are able to confirm that the patient is eligible for publicly funded pharmaceuticals or the prescriber is approved for co-payments.
Dispensing process

The dispensing process is a team effort, with pharmacy staff working together and within their scope of practice to provide customers safely and accurately with their prescribed medicines. Errors can have serious consequences, and it is vital that all members of the team carry out the required checks throughout each step of the process.
As a pharmacy technician, this will involve ensuring the right person is dispensed:
- the right medicine
- in the right dose and frequency
- in the right dose form
- the right quantity of supply
- the right information and advice
It also involves carrying out the right:
- checks
- computer input
- medicine labelling
- dispensing practices and procedures
How to dispense
This dispensing process is a generalised overview. The process in the pharmacy you work in may differ, as all pharmacies have their own SOPs, but all the same tasks will apply.
11 Step dispensing process
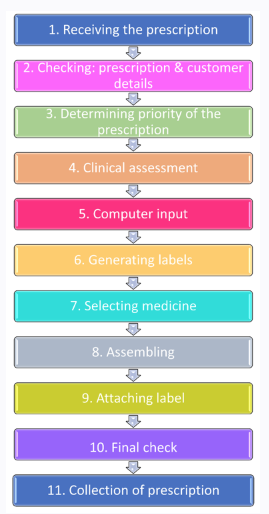
Now you have an overview of the dispensing process, it is time to dive into the details of each step in order.
STEP 1. RECEIVING THE PRESCRIPTION

From your previous pharmacy studies, you will be familiar with the beginning of the dispensing process.
Stat and non-stat dispensing
Medicines may be dispensed in two ways:
| Stat | A prescription for three months’ supply may be dispensed all at once |
| Non-stat | A prescription for three months’ supply can ONLY be dispensed one month at a time. |
The Pharmaceutical Schedule lists which medicines are stat and which are non-stat.
For patients with ongoing medication needs, their prescriber may write a prescription that includes a specified number of repeats for a non-stat medication. This means that the patient can collect one month's supply of their medicine and then return to the pharmacy to collect additional repeats of the prescription as needed. By having a set number of repeats, the patient can avoid having to return to their prescriber for each refill and can ensure they have a continuous supply of their medication.
A prescription form with repeats will look similar to this
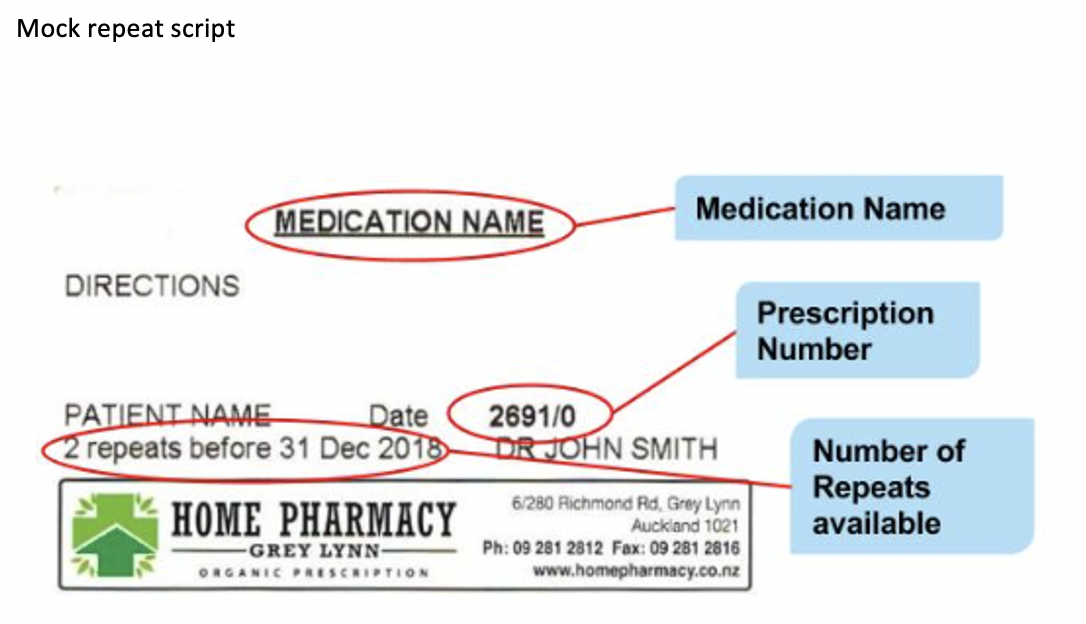
Sometimes, a prescriber or a customer may want to have a non-CD medicine that is non-stat, dispensed all at once (stat). Under the Pharmaceutical Schedules rules, this is allowed under the following circumstances:
- If the customer has limited mobility
- If the customer lives more than 30 minutes from the nearest pharmacy by their normal form of transport
- If the customer is re-locating to another area
- If the customer is travelling and will be away when the repeat prescriptions are due
If any of these circumstances apply, then the pharmacy can dispense the requested medicine stat. The pharmacy will stamp the prescription form with an access exemption stamp, which the customer signs and indicates the reason for the stat dispensing.
For controlled drugs to be dispensed stat, a doctor's permission is required.
Self-directed learning activities
Instructions:
- Read the following eight questions.
- Using your notes, resources, and the internet, carry out some research to help you answer the questions.
Questions:
- According to the Health Entitlement Cards Regulations, what is meant by the following:
- Partnered or married
- Child
- Dependent child
- Family unit
- If a married person is entitled to a CSC, will their spouse also get a card?
- Can a person’s CSC card be used for other people, for example a friend, family member, or dependent child?
- Are people who are holders of SuperGold or Veterans cards issued a CSC as well?
- Can an individual’s HUHC be used for other people, for example a friend, family member, or dependent child?
- What is a Veteran?
- What kind of card is issued to a Veteran with a Disability Pension?
- Are the following people charged a co-payment?
- Children under 14 years
- People receiving antibiotics through the Rheumatic Fever Prevention Programme
- People receiving Antituberculotic (TB) items on a prescription form
Whakamihi! Yes! Another week of Introduction to Dispensing completed!

Previously, we looked at tablets as one example of a solid dose form. We looked at the variety of tablet types and noted their advantages, disadvantages, and the customer advice that a pharmacy technician should provide when dispensing them. We will now spend some time looking at other solid dose forms.
Before we move on, let's examine two of the ways tablets can be taken:
- Watch (~1m): How To Swallow Tablets Easily - AbrahamThePharmacist (YouTube)
- Read: How to use suppositories on Healthify.
Other solid dose forms
Another type of solid dose form is implants.
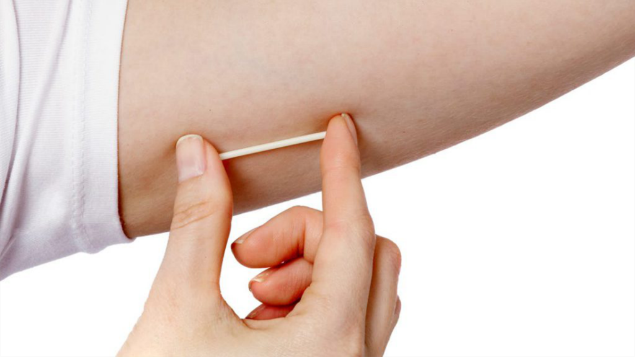
One type of implant is the contraceptive implant, which is often just referred to as ‘the implant’ or ‘the rod’.
- Read this page on Healthify to learn more about this dose form.
Research and document
Your learning activity is to carry out your own research and complete the following table about other solid dose forms.
Transdermal Patches
Transdermal patches are a dose form that can deliver a variety of medications by being absorbed through the skin. As a result of this method of delivery, the medication has a systemic effect on the body. You will be familiar with the use of nicotine patches in nicotine replacement therapy (NRT). To refresh your learning about NRT you may like to read and watch this short video clip on Healthify.
A transdermal patch typically has three layers.
Learn about these by expanding each of the labels or selecting the (+) signs below:
Some transdermal patches may also include additional layers, such as a protective release liner that covers the adhesive layer until the patch is ready to be applied or a membrane that controls the rate at which the medication is released.
Time for some more independent research! Answer these questions and record your answers in your journal.
Instructions:
Read the following three questions.
Questions:
- Other than for use in NRT, what other medications can be delivered via a transdermal patch?
- What are the advantages and disadvantages of this dose form?
- What advice would you give to a customer who is prescribed this dose form - what might they need to know about how to apply it, etc?
Self-directed learning activities
In today’s SDL, we continue our study on solid dose forms.
Instructions:
Read the following task.
Task:
Your task is to find out about:
- medicated lozenges
- sublingual tablets
- buccal tablets.
For each type of medication, provide an example (give the trade or generic name) and create a bullet-point list that includes:
- the purpose of the medicine and what it is used to treat
- the advantages and disadvantages of the dose form
- key points to advise customers on the proper use and storage of the medication.
Well done! You have completed another week of Patient Care!

